ROS, Thiols and Thiol-Regulating Systems in Male Gametogenesis☆
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Peroxiredoxins in Neurodegenerative Diseases
antioxidants Review Peroxiredoxins in Neurodegenerative Diseases Monika Szeliga Mossakowski Medical Research Centre, Department of Neurotoxicology, Polish Academy of Sciences, 5 Pawinskiego Street, 02-106 Warsaw, Poland; [email protected]; Tel.: +48-(22)-6086416 Received: 31 October 2020; Accepted: 27 November 2020; Published: 30 November 2020 Abstract: Substantial evidence indicates that oxidative/nitrosative stress contributes to the neurodegenerative diseases. Peroxiredoxins (PRDXs) are one of the enzymatic antioxidant mechanisms neutralizing reactive oxygen/nitrogen species. Since mammalian PRDXs were identified 30 years ago, their significance was long overshadowed by the other well-studied ROS/RNS defense systems. An increasing number of studies suggests that these enzymes may be involved in the neurodegenerative process. This article reviews the current knowledge on the expression and putative roles of PRDXs in neurodegenerative disorders such as Alzheimer’s disease, Parkinson’s disease and dementia with Lewy bodies, multiple sclerosis, amyotrophic lateral sclerosis and Huntington’s disease. Keywords: peroxiredoxin (PRDX); oxidative stress; nitrosative stress; neurodegenerative disease 1. Introduction Under physiological conditions, reactive oxygen species (ROS, e.g., superoxide anion, O2 -; · hydrogen peroxide, H O ; hydroxyl radical, OH; organic hydroperoxide, ROOH) and reactive nitrogen 2 2 · species (RNS, e.g., nitric oxide, NO ; peroxynitrite, ONOO-) are constantly produced as a result of normal · cellular metabolism and play a crucial role in signal transduction, enzyme activation, gene expression, and regulation of immune response [1]. The cells are endowed with several enzymatic (e.g., glutathione peroxidase (GPx); peroxiredoxin (PRDX); thioredoxin (TRX); catalase (CAT); superoxide dismutase (SOD)), and non-enzymatic (e.g., glutathione (GSH); quinones; flavonoids) antioxidant systems that minimize the levels of ROS and RNS. -

Surface Phenotype Changes and Increased Response to Oxidative Stress in CD4+Cd25high T Cells
biomedicines Article Surface Phenotype Changes and Increased Response to Oxidative Stress in CD4+CD25high T Cells Yoshiki Yamamoto 1,*, Takaharu Negoro 2, Rui Tada 3 , Michiaki Narushima 4, Akane Hoshi 2, Yoichi Negishi 3,* and Yasuko Nakano 2 1 Department of Paediatrics, Tokyo Metropolitan Ebara Hospital, Tokyo 145-0065, Japan 2 Department of Pharmacogenomics, School of Pharmacy, Showa University, Tokyo 142-8555, Japan; [email protected] (T.N.); [email protected] (A.H.); [email protected] (Y.N.) 3 Department of Drug Delivery and Molecular Biopharmaceutics, School of Pharmacy, Tokyo University of Pharmacy and Life Sciences, Tokyo 192-0392, Japan; [email protected] 4 Department of Internal Medicine, Showa University Northern Yokohama Hospital, Kanagawa 224-8503, Japan; [email protected] * Correspondence: [email protected] (Y.Y.); [email protected] (Y.N.); Tel.: +81-3-5734-8000 (Y.Y.); +81-42-676-3182 (Y.N.) + + + + Abstract: Conversion of CD4 CD25 FOXP3 T regulatory cells (Tregs) from the immature (CD45RA ) to mature (CD45RO+) phenotype has been shown during development and allergic reactions. The relative frequencies of these Treg phenotypes and their responses to oxidative stress during devel- opment and allergic inflammation were analysed in samples from paediatric and adult subjects. The FOXP3lowCD45RA+ population was dominant in early childhood, while the percentage of high + FOXP3 CD45RO cells began increasing in the first year of life. These phenotypic changes were observed in subjects with and without asthma. Further, there was a significant increase in phospho- Citation: Yamamoto, Y.; Negoro, T.; + high rylated ERK1/2 (pERK1/2) protein in hydrogen peroxide (H2O2)-treated CD4 CD25 cells in Tada, R.; Narushima, M.; Hoshi, A.; adults with asthma compared with those without asthma. -

(ER) Membrane Contact Sites (MCS) Uses Toxic Waste to Deliver Messages Edgar Djaha Yoboue1, Roberto Sitia1 and Thomas Simmen2
Yoboue et al. Cell Death and Disease (2018) 9:331 DOI 10.1038/s41419-017-0033-4 Cell Death & Disease REVIEW ARTICLE Open Access Redox crosstalk at endoplasmic reticulum (ER) membrane contact sites (MCS) uses toxic waste to deliver messages Edgar Djaha Yoboue1, Roberto Sitia1 and Thomas Simmen2 Abstract Many cellular redox reactions housed within mitochondria, peroxisomes and the endoplasmic reticulum (ER) generate hydrogen peroxide (H2O2) and other reactive oxygen species (ROS). The contribution of each organelle to the total cellular ROS production is considerable, but varies between cell types and also over time. Redox-regulatory enzymes are thought to assemble at a “redox triangle” formed by mitochondria, peroxisomes and the ER, assembling “redoxosomes” that sense ROS accumulations and redox imbalances. The redoxosome enzymes use ROS, potentially toxic by-products made by some redoxosome members themselves, to transmit inter-compartmental signals via chemical modifications of downstream proteins and lipids. Interestingly, important components of the redoxosome are ER chaperones and oxidoreductases, identifying ER oxidative protein folding as a key ROS producer and controller of the tri-organellar membrane contact sites (MCS) formed at the redox triangle. At these MCS, ROS accumulations could directly facilitate inter-organellar signal transmission, using ROS transporters. In addition, ROS influence the flux 2+ 2+ of Ca ions, since many Ca handling proteins, including inositol 1,4,5 trisphosphate receptors (IP3Rs), SERCA pumps or regulators of the mitochondrial Ca2+ uniporter (MCU) are redox-sensitive. Fine-tuning of these redox and ion signaling pathways might be difficult in older organisms, suggesting a dysfunctional redox triangle may accompany 1234567890 1234567890 the aging process. -
Lecture 7- PDF Drugs in Male Infertility .Pdf
By the end of this lecture you will be able to: Define male infertility Recognize regulations contributing to male fertility & dysregulations leading to infertility Classify hormonal & non-hormonal therapies used in male infertility whether being empirical or specific. Expand on the mechanism of action, indications, preparations, side effects, contraindications & interactions of most hormonal therapies Highlight some potentialities of emperical non-hormonal therapies MALE INFERTILITY Definition Inability of a male to achieve conception in a fertile woman after one year of unprotected intercourse. Prevalence Approximately 15-20% of all cohabiting couples are infertile In up to + 50% of such cases, males are responsible Mature Sperm / No motility / Sterile A Seminiferous Tubule Spermiation Site of SPERMATOGENESIS LEYDIG C. TESTOSTERONE Spermiogenesis Spermatidogenesis SERTOLI C. Spermato cytogenesis ~74 dys/ 15 dys in epididymis SPERMATOGONIUM Testosterone Secretion & Spermatogenesis Nests GERM CELLS the Are Coupled Communicate in a paracrine fashion LEYDIG C. TESTOSTERONE SERTOLI C. Separate them from rest of body by the blood-testicular barrier Converts Testosterone to Dihydrotestosterone [DHT] & Estradiol to direct spermatogenesis Secret androgen-binding-proteins [ABP] concentrate & testosterone in seminiferous tubules to stimulate spermiogenesis Steps from Spermatid to Spermatozoan Proceed Seminiferous ductsRete testis Efferent ductulesEpididymis DHT > Testosterone + Estradiol + other paracrine/autocrine [ Develop Motility & Fertilizing -

SUPPLEMENTARY DATA Supplementary Figure 1. The
SUPPLEMENTARY DATA Supplementary Figure 1. The results of Sirt1 activation in primary cultured TG cells using adenoviral system. GFP expression served as the control (n = 4 per group). Supplementary Figure 2. Two different Sirt1 activators, SRT1720 (0.5 µM or 1 µM ) and RSV (1µM or 10µM), induced the upregulation of Sirt1 in the primary cultured TG cells (n = 4 per group). ©2016 American Diabetes Association. Published online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db15-1283/-/DC1 SUPPLEMENTARY DATA Supplementary Table 1. Primers used in qPCR Gene Name Primer Sequences Product Size (bp) Sirt1 F: tgccatcatgaagccagaga 241 (NM_001159589) R: aacatcgcagtctccaagga NOX4 F: tgtgcctttattgtgcggag 172 (NM_001285833.1) R: gctgatacactggggcaatg Supplementary Table 2. Antibodies used in Western blot or Immunofluorescence Antibody Company Cat. No Isotype Dilution Sirt1 Santa Cruz * sc-15404 Rabbit IgG 1/200 NF200 Sigma** N5389 Mouse IgG 1/500 Tubulin R&D# MAB1195 Mouse IgG 1/500 NOX4 Abcam† Ab133303 Rabbit IgG 1/500 NOX2 Abcam Ab129068 Rabbit IgG 1/500 phospho-AKT CST‡ #4060 Rabbit IgG 1/500 EGFR CST #4267 Rabbit IgG 1/500 Ki67 Santa Cruz sc-7846 Goat IgG 1/500 * Santa Cruz Biotechnology, Santa Cruz, CA, USA ** Sigma aldrich, Shanghai, China # R&D Systems Inc, Minneapolis, MN, USA † Abcam, Inc., Cambridge, MA, USA ‡ Cell Signaling Technology, Inc., Danvers, MA, USA ©2016 American Diabetes Association. Published online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db15-1283/-/DC1 SUPPLEMENTARY DATA Supplementary -

The Prognostic Values of the Peroxiredoxins Family in Ovarian Cancer
Bioscience Reports (2018) 38 BSR20180667 https://doi.org/10.1042/BSR20180667 Research Article The prognostic values of the peroxiredoxins family in ovarian cancer Saisai Li, Xiaoli Hu, Miaomiao Ye and Xueqiong Zhu Department of Obstetrics and Gynecology, the Second Affiliated Hospital of Wenzhou Medical University, Wenzhou 325027, Zhejiang, China Correspondence: Xueqiong Zhu ([email protected]) Purpose: Peroxiredoxins (PRDXs) are a family of antioxidant enzymes with six identified mammalian isoforms (PRDX1–6). PRDX expression is up-regulated in various types of solid tumors; however, individual PRDX expression, and its impact on prognostic value in ovarian cancer patients, remains unclear. Methods: PRDXs family protein expression profiles in normal ovarian tissues and ovarian cancer tissues were examined using the Human Protein Atlas database. Then, the prog- nostic roles of PRDX family members in several sets of clinical data (histology, pathological grades, clinical stages, and applied chemotherapy) in ovarian cancer patients were investi- gated using the Kaplan–Meier plotter. Results: PRDXs family protein expression in ovarian cancer tissues was elevated com- pared with normal ovarian tissues. Meanwhile, elevated expression of PRDX3, PRDX5, and PRDX6 mRNAs showed poorer overall survival (OS); PRDX5 and PRDX6 also predicted poor progression-free survival (PFS) for ovarian cancer patients. Furthermore, PRDX3 played sig- nificant prognostic roles, particularly in poor differentiation and late-stage serous ovarian cancer patients. Additionally, PRDX5 predicted a lower PFS in all ovarian cancer patients treated with Platin, Taxol, and Taxol+Platin chemotherapy. PRDX3 and PRDX6 also showed poor PFS in patients treated with Platin chemotherapy. Furthermore, PRDX3 and PRDX5 indicated lower OS in patients treated with these three chemotherapeutic agents. -

GPX4 at the Crossroads of Lipid Homeostasis and Ferroptosis Giovanni C
REVIEW GPX4 www.proteomics-journal.com GPX4 at the Crossroads of Lipid Homeostasis and Ferroptosis Giovanni C. Forcina and Scott J. Dixon* formation of toxic radicals (e.g., R-O•).[5] Oxygen is necessary for aerobic metabolism but can cause the harmful The eight mammalian GPX proteins fall oxidation of lipids and other macromolecules. Oxidation of cholesterol and into three clades based on amino acid phospholipids containing polyunsaturated fatty acyl chains can lead to lipid sequence similarity: GPX1 and GPX2; peroxidation, membrane damage, and cell death. Lipid hydroperoxides are key GPX3, GPX5, and GPX6; and GPX4, GPX7, and GPX8.[6] GPX1–4 and 6 (in intermediates in the process of lipid peroxidation. The lipid hydroperoxidase humans) are selenoproteins that contain glutathione peroxidase 4 (GPX4) converts lipid hydroperoxides to lipid an essential selenocysteine in the enzyme + alcohols, and this process prevents the iron (Fe2 )-dependent formation of active site, while GPX5, 6 (in mouse and toxic lipid reactive oxygen species (ROS). Inhibition of GPX4 function leads to rats), 7, and 8 use an active site cysteine lipid peroxidation and can result in the induction of ferroptosis, an instead. Unlike other family members, GPX4 (PHGPx) can act as a phospholipid iron-dependent, non-apoptotic form of cell death. This review describes the hydroperoxidase to reduce lipid perox- formation of reactive lipid species, the function of GPX4 in preventing ides to lipid alcohols.[7,8] Thus,GPX4ac- oxidative lipid damage, and the link between GPX4 dysfunction, lipid tivity is essential to maintain lipid home- oxidation, and the induction of ferroptosis. ostasis in the cell, prevent the accumula- tion of toxic lipid ROS and thereby block the onset of an oxidative, iron-dependent, non-apoptotic mode of cell death termed 1. -

Role of Oxidative Stress and Nrf2/KEAP1 Signaling in Colorectal Cancer: Mechanisms and Therapeutic Perspectives with Phytochemicals
antioxidants Review Role of Oxidative Stress and Nrf2/KEAP1 Signaling in Colorectal Cancer: Mechanisms and Therapeutic Perspectives with Phytochemicals Da-Young Lee, Moon-Young Song and Eun-Hee Kim * College of Pharmacy and Institute of Pharmaceutical Sciences, CHA University, Seongnam 13488, Korea; [email protected] (D.-Y.L.); [email protected] (M.-Y.S.) * Correspondence: [email protected]; Tel.: +82-31-881-7179 Abstract: Colorectal cancer still has a high incidence and mortality rate, according to a report from the American Cancer Society. Colorectal cancer has a high prevalence in patients with inflammatory bowel disease. Oxidative stress, including reactive oxygen species (ROS) and lipid peroxidation, has been known to cause inflammatory diseases and malignant disorders. In particular, the nuclear factor erythroid 2-related factor 2 (Nrf2)/Kelch-like ECH-related protein 1 (KEAP1) pathway is well known to protect cells from oxidative stress and inflammation. Nrf2 was first found in the homolog of the hematopoietic transcription factor p45 NF-E2, and the transcription factor Nrf2 is a member of the Cap ‘N’ Collar family. KEAP1 is well known as a negative regulator that rapidly degrades Nrf2 through the proteasome system. A range of evidence has shown that consumption of phytochemicals has a preventive or inhibitory effect on cancer progression or proliferation, depending on the stage of colorectal cancer. Therefore, the discovery of phytochemicals regulating the Nrf2/KEAP1 axis and Citation: Lee, D.-Y.; Song, M.-Y.; verification of their efficacy have attracted scientific attention. In this review, we summarize the role Kim, E.-H. Role of Oxidative Stress of oxidative stress and the Nrf2/KEAP1 signaling pathway in colorectal cancer, and the possible and Nrf2/KEAP1 Signaling in utility of phytochemicals with respect to the regulation of the Nrf2/KEAP1 axis in colorectal cancer. -

Extracellular Vesicles Derived from Induced Pluripotent Stem Cells Promote Renoprotection in Acute Kidney Injury Model
cells Article Extracellular Vesicles Derived from Induced Pluripotent Stem Cells Promote Renoprotection in Acute Kidney Injury Model Federica Collino 1,2,3 , Jarlene A. Lopes 1,2,4, Marta Tapparo 5, Giovane G. Tortelote 1,6, Taís H. Kasai-Brunswick 1,2,4, Gustavo M.C. Lopes 1,2,4, Douglas B. Almeida 1,2,4, Renata Skovronova 7, Camila H. C. Wendt 1, Kildare R. de Miranda 1,4,8, Benedetta Bussolati 7 , Adalberto Vieyra 1,2,4,9,* and Rafael Soares Lindoso 1,2,4,* 1 Institute of Biophysics Carlos Chagas Filho, Federal University of Rio de Janeiro, 21941-902 Rio de Janeiro, Brazil; [email protected] (F.C.); [email protected] (J.A.L.); [email protected] (G.G.T.); [email protected] (T.H.K.-B.); [email protected] (G.M.C.L.); [email protected] (D.B.A.); [email protected] (C.H.C.W.); [email protected] (K.R.d.M.) 2 National Institute of Science and Technology for Regenerative Medicine-REGENERA, Federal University of Rio de Janeiro, 21941-902 Rio de Janeiro, Brazil 3 Department of Biomedical Sciences, University of Padova, 35131 Padua, Italy 4 National Center for Structural Biology and Bioimaging/CENABIO, Federal University of Rio de Janeiro, 21941-902 Rio de Janeiro, Brazil 5 Department of Medical Sciences, Molecular Biotechnology Center, University of Torino, 10126 Torino, Italy; [email protected] 6 Department of Pediatrics’ Section of Pediatric Nephrology, Tulane University School of Medicine, New Orleans, LA 70112, USA 7 Department of Molecular Biotechnology and Health Sciences, University of Torino, -
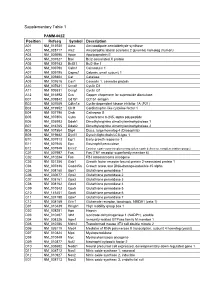
Supplementary Table 1
Supplementary Table 1 PAMM-062Z Position Refseq Symbol Description A01 NM_013930 Aass Aminoadipate-semialdehyde synthase A02 NM_028717 Als2 Amyotrophic lateral sclerosis 2 (juvenile) homolog (human) A03 NM_009696 Apoe Apolipoprotein E A04 NM_007527 Bax Bcl2-associated X protein A05 NM_009743 Bcl2l1 Bcl2-like 1 A06 NM_009790 Calm1 Calmodulin 1 A07 NM_009795 Capns1 Calpain, small subunit 1 A08 NM_009804 Cat Catalase A09 NM_007616 Cav1 Caveolin 1, caveolae protein A10 NM_007631 Ccnd1 Cyclin D1 A11 NM_009831 Ccng1 Cyclin G1 A12 NM_016892 Ccs Copper chaperone for superoxide dismutase B01 NM_009842 Cd151 CD151 antigen B02 NM_007669 Cdkn1a Cyclin-dependent kinase inhibitor 1A (P21) B03 NM_019952 Clcf1 Cardiotrophin-like cytokine factor 1 B04 NM_007798 Ctsb Cathepsin B B05 NM_007806 Cyba Cytochrome b-245, alpha polypeptide B06 NM_026993 Ddah1 Dimethylarginine dimethylaminohydrolase 1 B07 NM_016765 Ddah2 Dimethylarginine dimethylaminohydrolase 2 B08 NM_007864 Dlg4 Discs, large homolog 4 (Drosophila) B09 NM_019682 Dynll1 Dynein light chain LC8-type 1 B10 NM_007913 Egr1 Early growth response 1 B11 NM_007946 Epx Eosinophil peroxidase B12 NM_007949 Ercc2 Excision repair cross-complementing rodent repair deficiency, complementation group 2 C01 NM_007987 Fas Fas (TNF receptor superfamily member 6) C02 NM_010234 Fos FBJ osteosarcoma oncogene C03 NM_021356 Gab1 Growth factor receptor bound protein 2-associated protein 1 C04 NM_007836 Gadd45a Growth arrest and DNA-damage-inducible 45 alpha C05 NM_008160 Gpx1 Glutathione peroxidase 1 C06 NM_030677 Gpx2 Glutathione -

Synergic Crosstalk Between Inflammation, Oxidative Stress
antioxidants Review Synergic Crosstalk between Inflammation, Oxidative Stress, and Genomic Alterations in BCR–ABL-Negative Myeloproliferative Neoplasm Alessandro Allegra 1,* , Giovanni Pioggia 2 , Alessandro Tonacci 3 , Marco Casciaro 4 , Caterina Musolino 1 and Sebastiano Gangemi 4 1 Department of Human Pathology in Adulthood and Childhood “Gaetano Barresi”, Division of Hematology, University of Messina, 98125 Messina, Italy; [email protected] 2 Institute for Biomedical Research and Innovation (IRIB), National Research Council of Italy (CNR), 98164 Messina, Italy; [email protected] 3 Clinical Physiology Institute, National Research Council of Italy (IFC-CNR), 56124 Pisa, Italy; [email protected] 4 School and Operative Unit of Allergy and Clinical Immunology, Department of Clinical and Experimental Medicine, University of Messina, 98125 Messina, Italy; [email protected] (M.C.); [email protected] (S.G.) * Correspondence: [email protected]; Tel.: +39-09-0221-2364 Received: 2 September 2020; Accepted: 21 October 2020; Published: 23 October 2020 Abstract: Philadelphia-negative chronic myeloproliferative neoplasms (MPNs) have recently been revealed to be related to chronic inflammation, oxidative stress, and the accumulation of reactive oxygen species. It has been proposed that MPNs represent a human inflammation model for tumor advancement, in which long-lasting inflammation serves as the driving element from early tumor stage (over polycythemia vera) to the later myelofibrotic cancer stage. It has been theorized that the starting event for acquired stem cell alteration may occur after a chronic inflammation stimulus with consequent myelopoietic drive, producing a genetic stem cell insult. When this occurs, the clone itself constantly produces inflammatory components in the bone marrow; these elements further cause clonal expansion. -

Catalysis of Peroxide Reduction by Fast Reacting Protein Thiols Focus Review †,‡ †,‡ ‡,§ ‡,§ ∥ Ari Zeida, Madia Trujillo, Gerardo Ferrer-Sueta, Ana Denicola, Darío A
Review Cite This: Chem. Rev. 2019, 119, 10829−10855 pubs.acs.org/CR Catalysis of Peroxide Reduction by Fast Reacting Protein Thiols Focus Review †,‡ †,‡ ‡,§ ‡,§ ∥ Ari Zeida, Madia Trujillo, Gerardo Ferrer-Sueta, Ana Denicola, Darío A. Estrin, and Rafael Radi*,†,‡ † ‡ § Departamento de Bioquímica, Centro de Investigaciones Biomedicaś (CEINBIO), Facultad de Medicina, and Laboratorio de Fisicoquímica Biologica,́ Facultad de Ciencias, Universidad de la Republica,́ 11800 Montevideo, Uruguay ∥ Departamento de Química Inorganica,́ Analítica y Química-Física and INQUIMAE-CONICET, Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires, 2160 Buenos Aires, Argentina ABSTRACT: Life on Earth evolved in the presence of hydrogen peroxide, and other peroxides also emerged before and with the rise of aerobic metabolism. They were considered only as toxic byproducts for many years. Nowadays, peroxides are also regarded as metabolic products that play essential physiological cellular roles. Organisms have developed efficient mechanisms to metabolize peroxides, mostly based on two kinds of redox chemistry, catalases/peroxidases that depend on the heme prosthetic group to afford peroxide reduction and thiol-based peroxidases that support their redox activities on specialized fast reacting cysteine/selenocysteine (Cys/Sec) residues. Among the last group, glutathione peroxidases (GPxs) and peroxiredoxins (Prxs) are the most widespread and abundant families, and they are the leitmotif of this review. After presenting the properties and roles of different peroxides in biology, we discuss the chemical mechanisms of peroxide reduction by low molecular weight thiols, Prxs, GPxs, and other thiol-based peroxidases. Special attention is paid to the catalytic properties of Prxs and also to the importance and comparative outlook of the properties of Sec and its role in GPxs.