Jco3615newview 1..1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Monoclonal Antibody: a New Treatment Strategy Against Multiple Myeloma
antibodies Review Monoclonal Antibody: A New Treatment Strategy against Multiple Myeloma Shih-Feng Cho 1,2,3, Liang Lin 3, Lijie Xing 3,4, Tengteng Yu 3, Kenneth Wen 3, Kenneth C. Anderson 3 and Yu-Tzu Tai 3,* 1 Division of Hematology & Oncology, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung 807, Taiwan; [email protected] 2 Faculty of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung 807, Taiwan 3 LeBow Institute for Myeloma Therapeutics and Jerome Lipper Multiple Myeloma Center, Dana-Farber Cancer Institute, Harvard Medical School, Boston, MA 02215, USA; [email protected] (L.L.); [email protected] (L.X.); [email protected] (T.Y.); [email protected] (K.W.); [email protected] (K.C.A.) 4 Department of Hematology, Shandong Provincial Hospital Affiliated to Shandong University, No. 324, Jingwu Road, Jinan 250021, China * Correspondence: [email protected]; Tel.: +1-617-632-3875; Fax: +1-617-632-2140 Received: 20 October 2017; Accepted: 10 November 2017; Published: 14 November 2017 Abstract: 2015 was a groundbreaking year for the multiple myeloma community partly due to the breakthrough approval of the first two monoclonal antibodies in the treatment for patients with relapsed and refractory disease. Despite early disappointments, monoclonal antibodies targeting CD38 (daratumumab) and signaling lymphocytic activation molecule F7 (SLAMF7) (elotuzumab) have become available for patients with multiple myeloma in the same year. Specifically, phase 3 clinical trials of combination therapies incorporating daratumumab or elotuzumab indicate both efficacy and a very favorable toxicity profile. These therapeutic monoclonal antibodies for multiple myeloma can kill target cells via antibody-dependent cell-mediated cytotoxicity, complement-dependent cytotoxicity, and antibody-dependent phagocytosis, as well as by direct blockade of signaling cascades. -

Blincyto Pi Hcp English.Pdf
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use BLINCYTO® safely and effectively. See full prescribing information for BLINCYTO. - Premedicate with dexamethasone. (2.2) BLINCYTO® (blinatumomab) for injection, for intravenous use • Refer to Full Prescribing Information for important preparation and Initial U.S. Approval: 2014 administration information. (2.4, 2.5, 2.6) • Administer as a continuous intravenous infusion at a constant flow rate using an infusion pump. (2.5, 2.6) WARNING: CYTOKINE RELEASE SYNDROME and - See Section 2.5 for infusion over 24 hours or 48 hours. NEUROLOGICAL TOXICITIES - See Section 2.6 for infusion over 7 days using Bacteriostatic 0.9% See full prescribing information for complete boxed warning. Sodium Chloride Injection, USP (containing 0.9% benzyl alcohol). This option is not recommended for patients weighing less than 22 kg. • Cytokine Release Syndrome (CRS), which may be life-threatening or fatal, occurred in patients receiving BLINCYTO. Interrupt or ---------------------DOSAGE FORMS AND STRENGTHS---------------------- discontinue BLINCYTO and treat with corticosteroids as For injection: 35 mcg of lyophilized powder in a single-dose vial for recommended. (2.3, 5.1) reconstitution. (3) • Neurological toxicities, which may be severe, life-threatening, or fatal, occurred in patients receiving BLINCYTO. Interrupt or discontinue -------------------------------CONTRAINDICATIONS------------------------------ BLINCYTO as recommended. (2.3, 5.2) Known hypersensitivity -

The Potential of Adoptive Transfer of Γ9δ2 T Cells to Enhance
www.nature.com/scientificreports OPEN The potential of adoptive transfer of γ9δ2 T cells to enhance blinatumomab’s antitumor activity against B‑cell malignancy Yun‑Hsiang Chen1, Yun Wang2, Cheng‑Hao Liao3 & Shu‑Ching Hsu4,5,6,7,8,9* Blinatumomab, a bispecifc T cell engager (BiTE) antibody targeting CD19 and CD3ε, can redirect T cells toward CD19‑positive tumor cells and has been approved to treat relapsed/refractory B‑cell acute lymphoblastic leukemia (R/R B‑ALL). However, chemotherapeutic regimens can severely reduce T cells’ number and cytotoxic function, leading to an inadequate response to blinatumomab treatment in patients. In addition, it was reported that a substantial portion of R/R B‑ALL patients failing blinatumomab treatment had the extramedullary disease, indicating the poor ability of blinatumomab in treating extramedullary disease. In this study, we investigated whether the adoptive transfer of ex vivo expanded γ9δ2 T cells could act as the efector of blinatumomab to enhance blinatumomab’s antitumor activity against B‑cell malignancies in vivo. Repeated infusion of blinatumomab and human γ9δ2 T cells led to more prolonged survival than that of blinatumomab or human γ9δ2 T cells alone in the mice xenografted with Raji cells. Furthermore, adoptive transfer of γ9δ2 T cells reduced tumor mass outside the bone marrow, indicating the potential of γ9δ2 T cells to eradicate the extramedullary disease. Our results suggest that the addition of γ9δ2 T cells to the blinatumomab treatment regimens could be an efective approach to enhancing blinatumomab’s therapeutic efcacy. The concept of this strategy may also be applied to other antigen‑specifc BiTE therapies for other malignancies. -

Antibody–Drug Conjugates
Published OnlineFirst April 12, 2019; DOI: 10.1158/1078-0432.CCR-19-0272 Review Clinical Cancer Research Antibody–Drug Conjugates: Future Directions in Clinical and Translational Strategies to Improve the Therapeutic Index Steven Coats1, Marna Williams1, Benjamin Kebble1, Rakesh Dixit1, Leo Tseng1, Nai-Shun Yao1, David A. Tice1, and Jean-Charles Soria1,2 Abstract Since the first approval of gemtuzumab ozogamicin nism of activity of the cytotoxic warhead. However, the (Mylotarg; Pfizer; CD33 targeted), two additional antibody– enthusiasm to develop ADCs has not been dampened; drug conjugates (ADC), brentuximab vedotin (Adcetris; Seat- approximately 80 ADCs are in clinical development in tle Genetics, Inc.; CD30 targeted) and inotuzumab ozogami- nearly 600 clinical trials, and 2 to 3 novel ADCs are likely cin (Besponsa; Pfizer; CD22 targeted), have been approved for to be approved within the next few years. While the hematologic cancers and 1 ADC, trastuzumab emtansine promise of a more targeted chemotherapy with less tox- (Kadcyla; Genentech; HER2 targeted), has been approved to icity has not yet been realized with ADCs, improvements treat breast cancer. Despite a clear clinical benefit being dem- in technology combined with a wealth of clinical data are onstrated for all 4 approved ADCs, the toxicity profiles are helping to shape the future development of ADCs. In this comparable with those of standard-of-care chemotherapeu- review, we discuss the clinical and translational strategies tics, with dose-limiting toxicities associated with the mecha- associated with improving the therapeutic index for ADCs. Introduction in antibody, linker, and warhead technologies in significant depth (2, 3, 8, 9). Antibody–drug conjugates (ADC) were initially designed to leverage the exquisite specificity of antibodies to deliver targeted potent chemotherapeutic agents with the intention of improving Overview of ADCs in Clinical Development the therapeutic index (the ratio between the toxic dose and the Four ADCs have been approved over the last 20 years (Fig. -

High Risk Therapy Made Easy: Supporting High Risk Patients Through Complex Therapy
8/21/2018 High Risk Therapy Made Easy: Supporting high risk patients through complex therapy Lori Ranney, MSN, APRN, CPNP, CPHON Mylynda Livingston, MSN, APRN, AC PC-PNP, CPON Teresa Herriage, DNP, APRN, CPNP, CPHON Children’s Minnesota Disclaimers and Confidentiality Protections Children’s Minnesota makes no representations or warranties about the accuracy, reliability, or completeness of the content. Content is provided “as is” and is for informational use only. It is not a substitute for professional medical advice, diagnosis, or treatment. Children’s disclaims all warranties, express or implied, statutory or otherwise, including without limitation the implied warranties of merchantability, non-infringement of third parties’ rights, and fitness for a particular purpose. This content was developed for use in Children’s patient care environment and may not be suitable for use in other patient care environments. Children’s does not endorse, certify, or assess third parties’ competency. You hold all responsibility for your use or nonuse of the content. Children’s shall not be liable for claims, losses, or damages arising from or related to any use or misuse of the content. This content and its related discussions are privileged and confidential under Minnesota’s peer review statute (Minn. Stat. § 145.61 et. seq.). Do not disclose unless appropriately authorized. Notwithstanding the foregoing, content may be subject to copyright or trademark law; use of such information requires Children’s permission. This content may include patient protected health information. You agree to comply with all applicable state and federal laws protecting patient privacy and security including the Minnesota Health Records Act and the Health Insurance Portability and Accountability Act and its implementing regulations as amended from time to time. -

Genmab and Seattle Genetics Present Data from Tisotumab Vedotin Innovatv 204 Pivotal Trial in Recurrent Or Metastatic Cervical Cancer at ESMO Virtual Congress 2020
Genmab and Seattle Genetics Present Data from Tisotumab Vedotin innovaTV 204 Pivotal Trial in Recurrent or Metastatic Cervical Cancer at ESMO Virtual Congress 2020 Media Release COPENHAGEN, Denmark and BOTHELL, Wash., 21 September 2020 • Data featured in late-breaking proffered paper oral presentation • Biologics license application submission planned to support accelerated approval pathway with the FDA Genmab A/S (Nasdaq: GMAB) and Seattle Genetics, Inc. (Nasdaq: SGEN) today presented data from the innovaTV 204 pivotal phase 2, single-arm clinical trial evaluating tisotumab vedotin as monotherapy in patients with previously treated recurrent and/or metastatic cervical cancer at the European Society for Medical Oncology (ESMO) Virtual Congress 2020. Patients had previously received a doublet chemotherapy and, if eligible, bevacizumab as first-line therapy. Results from the trial showed a 24 percent confirmed objective response rate (ORR) by independent central review with a median duration of response (DOR) of 8.3 months. The most common treatment-related adverse events (greater than or equal to 20 percent) included alopecia, epistaxis (nose bleeds), nausea, conjunctivitis, fatigue and dry eye. Tisotumab vedotin is an investigational antibody-drug conjugate (ADC) directed to tissue factor (TF), which is prevalent on solid tumors including cervical cancer and can promote tumor growth, angiogenesis and metastasis.1 Current therapies for previously treated recurrent and/or metastatic cervical cancer generally result in limited objective response rates of typically less than 15 percent with median overall survival ranging from 6.0 to 9.4 months.1-8 “Following resistance to or progression on first-line standard of care therapy, there are limited treatment options for women with metastatic cervical cancer,” said Robert L. -
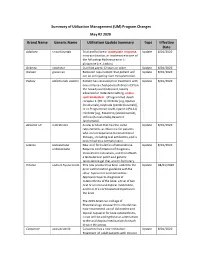
Summary of Utilization Management (UM) Program Changes May #2 2020
Summary of Utilization Management (UM) Program Changes May #2 2020 Brand Name Generic Name Utilization Update Summary Type Effective Date Adakveo crizanlizumab Trial and failure or inadequate response, Update 8/01/2020 contraindication, or intolerance to one of the following: Hydroxyurea or L- glutamine (i.e., Endari) Oxbryta voxelotor Clarified age to 12 years or older Update 8/01/2020 Givlaari givosiran Removed requirement that patient will Update 8/01/2020 not be anticipating liver transplantation. Padcev enfortumab vedotin Patient has received prior treatment with Update 8/01/2020 one immune checkpoint inhibitors (CPI) in the neoadjuvant/adjuvant, locally advanced or metastatic setting, unless contraindicated: i) Programmed death receptor-1 (PD-1) inhibitor [e.g.,Opdivo (nivolumab), Keytruda (pembrolizumab)], or ii) Programmed death-ligand 1 (PD-L1) inhibitor [e.g., Tecentriq (atezolizumab), Imfinzi (durvalumab), Bavencio (avelumab)]. Absorica LD isotretinoin A new product that has the same Update 8/01/2020 requirements as Absorica: for patients who are unresponsive to conventional therapy, including oral antibiotics, and is prescribed by a dermatologist. Jatenzo testosterone New oral formulation of testosterone. Update 8/01/2020 undecanoate Requires confirmation of diagnosis, testosterone lab values, and trial of both a testosterone patch and generic testosterone gel that are on formulary. Triluron sodium hyaluronate This new product has been added to the Update 08/01/2020 prior authorization guideline with the other hyaluronic acid derivatives. Approval requires diagnosis of osteoarthritis of the knee, a trial of two oral or an oral and topical medication, and trial of a corticosteroid injection in the knee. The 2019 American College of Rheumatology Osteoarthritis Guidelines now recommend use of duloxetine and topical capsaicin for knee osteoarthritis, so we will be adding these as alternatives to the oral/topical medications for each drug in this group. -

CDER Breakthrough Therapy Designation Approvals Data As of December 31, 2020 Total of 190 Approvals
CDER Breakthrough Therapy Designation Approvals Data as of December 31, 2020 Total of 190 Approvals Submission Application Type and Proprietary Approval Use Number Number Name Established Name Applicant Date Treatment of patients with previously BLA 125486 ORIGINAL-1 GAZYVA OBINUTUZUMAB GENENTECH INC 01-Nov-2013 untreated chronic lymphocytic leukemia in combination with chlorambucil Treatment of patients with mantle cell NDA 205552 ORIGINAL-1 IMBRUVICA IBRUTINIB PHARMACYCLICS LLC 13-Nov-2013 lymphoma (MCL) Treatment of chronic hepatitis C NDA 204671 ORIGINAL-1 SOVALDI SOFOSBUVIR GILEAD SCIENCES INC 06-Dec-2013 infection Treatment of cystic fibrosis patients age VERTEX PHARMACEUTICALS NDA 203188 SUPPLEMENT-4 KALYDECO IVACAFTOR 21-Feb-2014 6 years and older who have mutations INC in the CFTR gene Treatment of previously untreated NOVARTIS patients with chronic lymphocytic BLA 125326 SUPPLEMENT-60 ARZERRA OFATUMUMAB PHARMACEUTICALS 17-Apr-2014 leukemia (CLL) for whom fludarabine- CORPORATION based therapy is considered inappropriate Treatment of patients with anaplastic NOVARTIS lymphoma kinase (ALK)-positive NDA 205755 ORIGINAL-1 ZYKADIA CERITINIB 29-Apr-2014 PHARMACEUTICALS CORP metastatic non-small cell lung cancer (NSCLC) who have progressed on or are intolerant to crizotinib Treatment of relapsed chronic lymphocytic leukemia (CLL), in combination with rituximab, in patients NDA 206545 ORIGINAL-1 ZYDELIG IDELALISIB GILEAD SCIENCES INC 23-Jul-2014 for whom rituximab alone would be considered appropriate therapy due to other co-morbidities -

5.01.582 Antibody-Drug Conjugates
MEDICAL POLICY – 5.01.582 Antibody-Drug Conjugates Effective Date: June 1, 2021 RELATED MEDICAL POLICIES: Last Revised: May 11, 2021 None Replaces: N/A Select a hyperlink below to be directed to that section. POLICY CRITERIA | CODING | RELATED INFORMATION EVIDENCE REVIEW | REFERENCES | HISTORY ∞ Clicking this icon returns you to the hyperlinks menu above. Introduction An antibody is a blood protein. When the immune system detects an unhealthy cell, antibodies link to a molecule, known as an antigen, on the unhealthy cell. Monoclonal antibodies are produced in a laboratory. They are made to link to antigens usually found in high numbers on cancer cells. Antibody-drug conjugates combine monoclonal antibodies with certain chemotherapy drugs. The monoclonal antibodies find the cancer cells and the chemotherapy drug is released directly into those cells. The goal with this treatment is to target only cancer cells and spare nearby healthy cells. This policy describes when specific antibody-drug conjugates may be considered medically necessary. Note: The Introduction section is for your general knowledge and is not to be taken as policy coverage criteria. The rest of the policy uses specific words and concepts familiar to medical professionals. It is intended for providers. A provider can be a person, such as a doctor, nurse, psychologist, or dentist. A provider also can be a place where medical care is given, like a hospital, clinic, or lab. This policy informs them about when a service may be covered. Policy Coverage Criteria Drug Medical -

A Novel Therapy for Lymphoma Using T-Cell Bispecific Antibodies Ajay Prakash1 and Catherine S
Author Manuscript Published OnlineFirst on June 8, 2018; DOI: 10.1158/1078-0432.CCR-18-1363 Author manuscripts have been peer reviewed and accepted for publication but have not yet been edited. Immunity War: A Novel Therapy for Lymphoma Using T-cell Bispecific Antibodies Ajay Prakash1 and Catherine S. Diefenbach1 1. NYU Perlmutter Cancer Center, Department of Hematology-Oncology Corresponding Author: Catherine Diefenbach; NYU Perlmutter Cancer Center; 240 East 38th Street, 19th Floor, NY NY 10016; 212 731-5670; email: [email protected] Disclosures: CD: Research funding, Consulting Genentech Running title: A Novel Lymphoma Therapy Using T-cell Bispecific Antibodies Summary The activity of T cell mediated immunotherapies in B cell lymphoma has been limited to date. The novel bispecific antibody CD20-TCB, has a 2:1 antibody design to maximize T cell engagement, and demonstrates activity in preclinical models. This may represent a novel therapeutic approach for patients with relapsed/refractory NHL. Main Text In this issue of Clinical Cancer Research, Bacac and colleagues investigate the pre-clinical efficacy of a bispecific antibody in the treatment of lymphoma (1). To date, immunotherapy for the treatment of non-Hodgkin lymphoma (NHL) has been primarily focused on monoclonal antibody therapy, which has demonstrated both single agent activity and substantial synergy with cytotoxic chemotherapy (2). Further development of immunotherapy has been mixed. Chimeric antigen (CAR) T-cell therapy has demonstrated exciting activity with significant potential toxicities, and some preliminary successes have been seen with antibody drug conjugates. However, checkpoint blockade and other novel approaches have been complicated by disappointing activity as well as off-target side effects. -

Blinatumomab (Blincyto) When It Is Determined to Be Medically Necessary Because the Medical Criteria and Guidelines Shown Below Are Met
Corporate Medical Policy Blinatumomab (Blincyto ®) File Name: blinatumomab_blincyto Origination: 9/2017 Last CAP Review: 11/2018 Next CAP Review: 11/2019 Last Review: 11/2018 Description of Procedure or Service ® Blincyto is a bispecific CD19-directed CD3 T-cell engager indicated for the treatment of relapsed or refractory B-cell precursor acute lymphoblastic leukemia (ALL) in adults and children. ***Note: This Medical Policy is complex and technical. For questions concerning the technical language and/or specific clinical indications for its use, please consult your physician. Policy BCBSNC will provide coverage for Blinatumomab (Blincyto) when it is determined to be medically necessary because the medical criteria and guidelines shown below are met. Benefits Application This medical policy relates only to the services or supplies described herein. Please refer to the Member's Benefit Booklet for availability of benefits. Member's benefits may vary according to benefit design; therefore member benefit language should be reviewed before applying the terms of this medical policy. When Blinatumomab (Blincyto) is covered Blinatumomab (Blincyto) is considered medically necessary for the treatment of patients with relapsed or refractory B-cell precursor acute lymphoblastic leukemia (ALL). Use of Blinatumomab (Blincyto) may be considered medically necessary for clinical indications not listed above when the drug is prescribed for the treatment of cancer either: • In accordance with FDA label (when clinical benefit has been established, see Policy Guidelines); OR • In accordance with specific strong endorsement or support by nationally recognized compendia, when such recommendation is based on strong/high levels of evidence, and/or uniform consensus of clinical appropriateness has been reached. -

Antibodies for the Treatment of Brain Metastases, a Dream Or a Reality?
pharmaceutics Review Antibodies for the Treatment of Brain Metastases, a Dream or a Reality? Marco Cavaco, Diana Gaspar, Miguel ARB Castanho * and Vera Neves * Instituto de Medicina Molecular, Faculdade de Medicina, Universidade de Lisboa, Av. Prof. Egas Moniz, 1649-028 Lisboa, Portugal * Correspondence: [email protected] (M.A.R.B.C.); [email protected] (V.N.) Received: 19 November 2019; Accepted: 28 December 2019; Published: 13 January 2020 Abstract: The incidence of brain metastases (BM) in cancer patients is increasing. After diagnosis, overall survival (OS) is poor, elicited by the lack of an effective treatment. Monoclonal antibody (mAb)-based therapy has achieved remarkable success in treating both hematologic and non-central-nervous system (CNS) tumors due to their inherent targeting specificity. However, the use of mAbs in the treatment of CNS tumors is restricted by the blood–brain barrier (BBB) that hinders the delivery of either small-molecules drugs (sMDs) or therapeutic proteins (TPs). To overcome this limitation, active research is focused on the development of strategies to deliver TPs and increase their concentration in the brain. Yet, their molecular weight and hydrophilic nature turn this task into a challenge. The use of BBB peptide shuttles is an elegant strategy. They explore either receptor-mediated transcytosis (RMT) or adsorptive-mediated transcytosis (AMT) to cross the BBB. The latter is preferable since it avoids enzymatic degradation, receptor saturation, and competition with natural receptor substrates, which reduces adverse events. Therefore, the combination of mAbs properties (e.g., selectivity and long half-life) with BBB peptide shuttles (e.g., BBB translocation and delivery into the brain) turns the therapeutic conjugate in a valid approach to safely overcome the BBB and efficiently eliminate metastatic brain cells.