Unsaturated Fatty Acids Are Required for Continuous Proliferation of Transformed Androgen-Dependent Cells by Fibroblast Growth Factor Family Proteinsi
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

CHAPTER 29 ORGANIC CHEMICALS VI 29-1 Notes 1
)&f1y3X CHAPTER 29 ORGANIC CHEMICALS VI 29-1 Notes 1. Except where the context otherwise requires, the headings of this chapter apply only to: (a) Separate chemically defined organic compounds, whether or not containing impurities; (b) Mixtures of two or more isomers of the same organic compound (whether or not containing impurities), except mixtures of acyclic hydrocarbon isomers (other than stereoisomers), whether or not saturated (chapter 27); (c) The products of headings 2936 to 2939 or the sugar ethers and sugar esters, and their salts, of heading 2940, or the products of heading 2941, whether or not chemically defined; (d) Products mentioned in (a), (b) or (c) above dissolved in water; (e) Products mentioned in (a), (b) or (c) above dissolved in other solvents provided that the solution constitutes a normal and necessary method of putting up these products adopted solely for reasons of safety or for transport and that the solvent does not render the product particularly suitable for specific use rather than for general use; (f) The products mentioned in (a), (b), (c), (d) or (e) above with an added stabilizer (including an anticaking agent) necessary for their preservation or transport; (g) The products mentioned in (a), (b), (c), (d), (e) or (f) above with an added antidusting agent or a coloring or odoriferous substance added to facilitate their identification or for safety reasons, provided that the additions do not render the product particularly suitable for specific use rather than for general use; (h) The following products, diluted to standard strengths, for the production of azo dyes: diazonium salts, couplers used for these salts and diazotizable amines and their salts. -

Ricini Oleum
PHARMACOGNOSY II PHAR306 6th Semester 5th Lecture Prof. Dr. Müberra Koşar Ass. Prof. Dr. Aybike Yektaoğlu Eastern Mediterranean University Faculty of Pharmacy Department of Pharmacognosy PHARMACEUTICAL FIXED OILS AND ANIMAL FATS FIXED OILS & ANIMAL FATS Amygdalae oleum • “Almond oil” • obtained by crushing of the seeds of two varieties Prunus dulcis var. dulcis or P. dulcis var. amara (Rosaceae) in the cold • Almond oil is obtained in the Mediterranean countries (Italy, France, Spain and North Africa) where its culture is obtained • The only difference between the two varieties is the cyanogenic glycoside content of the var. amara FIXED OILS&ANIMAL FATS Amygdalae oleum • seeds carries 40-55% fixed oil • the refined oil mainly contains oleic acid (62-86%), linoleic (20- 30%), palmitic (4-9%) • Amydalae oleum raffinatum (Almond oil, refined) (Eur.Pu.) • Amydalae oleum virginale (Almond oil, virgin) (Eur.Ph.) • major used in cosmetology and dermatology • used as a carrier in oily injectable preparations FIXED OILS&ANIMAL FATS Arachidis oleum • “Arachis oil, Peanut oil” – “Peanut butter” • Arachis hypogaea (Fabaceae) • cultivated in South America, China, India, Australia, and West Africa • due to various genotypes they vary in fatty acid content • the seeds are cold-pressed • they have similar properties as olive oil • most suitable oil for added for embedding purposes into other oils (e.g. olive oil) FIXED OILS&ANIMAL FATS Arachidis oleum - content • seeds carries 40-50% fixed oil • 50-65% oleic acid • 18-30% linoleic acid • 8-10% palmitic -

The Natural Compounds Atraric Acid and N-Butylbenzene-Sulfonamide
The natural compounds atraric acid and N-butylbenzene-sulfonamide as antagonists of the human androgen receptor and inhibitors of prostate cancer cell growth Daniela Roell, Aria Baniahmad To cite this version: Daniela Roell, Aria Baniahmad. The natural compounds atraric acid and N-butylbenzene-sulfonamide as antagonists of the human androgen receptor and inhibitors of prostate cancer cell growth. Molec- ular and Cellular Endocrinology, Elsevier, 2010, 332 (1-2), pp.1. 10.1016/j.mce.2010.09.013. hal- 00654968 HAL Id: hal-00654968 https://hal.archives-ouvertes.fr/hal-00654968 Submitted on 24 Dec 2011 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Accepted Manuscript Title: The natural compounds atraric acid and N-butylbenzene-sulfonamide as antagonists of the human androgen receptor and inhibitors of prostate cancer cell growth Authors: Daniela Roell, Aria Baniahmad PII: S0303-7207(10)00474-0 DOI: doi:10.1016/j.mce.2010.09.013 Reference: MCE 7644 To appear in: Molecular and Cellular Endocrinology Received date: 29-6-2010 Revised date: 3-9-2010 Accepted date: 27-9-2010 Please cite this article as: Roell, D., Baniahmad, A., The natural compounds atraric acid and N-butylbenzene-sulfonamide as antagonists of the human androgen receptor and inhibitors of prostate cancer cell growth, Molecular and Cellular Endocrinology (2010), doi:10.1016/j.mce.2010.09.013 This is a PDF file of an unedited manuscript that has been accepted for publication. -

Annex 2B Tariff Schedule of the United States See General Notes to Annex 2B for Staging Explanation HTSUS No
Annex 2B Tariff Schedule of the United States See General Notes to Annex 2B for Staging Explanation HTSUS No. Description Base Rate Staging 0101 Live horses, asses, mules and hinnies: 0101.10.00 -Purebred breeding animals Free E 0101.90 -Other: 0101.90.10 --Horses Free E 0101.90.20 --Asses 6.8% B --Mules and hinnies: 0101.90.30 ---Imported for immediate slaughter Free E 0101.90.40 ---Other 4.5% A 0102 Live bovine animals: 0102.10.00 -Purebred breeding animals Free E 0102.90 -Other: 0102.90.20 --Cows imported specially for dairy purposes Free E 0102.90.40 --Other 1 cent/kg A 0103 Live swine: 0103.10.00 -Purebred breeding animals Free E -Other: 0103.91.00 --Weighing less than 50 kg each Free E 0103.92.00 --Weighing 50 kg or more each Free E 0104 Live sheep and goats: 0104.10.00 -Sheep Free E 0104.20.00 -Goats 68 cents/head A 0105 Live poultry of the following kinds: Chickens, ducks, geese, turkeys and guineas: -Weighing not more than 185 g: 0105.11.00 --Chickens 0.9 cents each A 0105.12.00 --Turkeys 0.9 cents each A 0105.19.00 --Other 0.9 cents each A -Other: 0105.92.00 --Chickens, weighing not more than 2,000 g 2 cents/kg A 0105.93.00 --Chickens, weighing more than 2,000 g 2 cents/kg A 0105.99.00 --Other 2 cents/kg A 0106 Other live animals: -Mammals: 0106.11.00 --Primates Free E 0106.12.00 --Whales, dolphins and porpoises (mammals of the order Cetacea); manatees and dugongs (mammals of the order Sirenia) Free E 0106.19 --Other: 2B-Schedule-1 HTSUS No. -
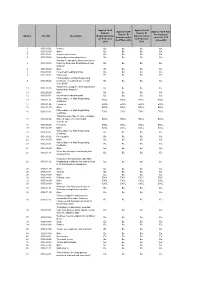
Number HS 2007 Description Applied Tariff Rate for Guatemala Under The
Applied Tariff Applied Tariff Applied Tariff Applied Tariff Rate Rate for Rate for El Rate for El for Honduras Number HS 2007 Description Guatemala under Salvador under Salvador under under the FTA the FTA since the FTA since the FTA in 2010 since 2010 2010 2011 1 0101.10.01 Horses Ex. Ex. Ex. Ex. 2 0101.10.99 Other Ex. Ex. Ex. Ex. 3 0101.90.01 Jump or race horses Ex. Ex. Ex. Ex. 4 0101.90.02 Non pedigreed breeding horses Ex. Ex. Ex. Ex. "Horses for slaughter, when imported by 5 0101.90.03 Federally Inspected Establishment type Ex. Ex. Ex. Ex. packers." 6 0101.90.99 Other Ex. Ex. Ex. Ex. 7 0102.10.01 Pure-bred breeding animals Ex. Ex. Ex. Ex. 8 0102.90.01 Dairy cows Ex. Ex. Ex. Ex. "With pedigree or High Registrating 9 0102.90.02 Certificate, excluding those of code Ex. Ex. Ex. Ex. 0102.90.01" "Bovines for slaughter, when imported by 10 0102.90.03 Ex. Ex. Ex. Ex. Industrial de Abastos." 11 0102.90.99 Other Ex. Ex. Ex. Ex. 12 0103.10.01 Pure-bred breeding animals Ex. Ex. Ex. Ex. With pedigree or High Registrating 13 0103.91.01 EXCL. EXCL. EXCL. EXCL. Certificate. 14 0103.91.02 Peccaries EXCL. EXCL. EXCL. EXCL. 15 0103.91.99 Other EXCL. EXCL. EXCL. EXCL. With pedigree or High Registrating 16 0103.92.01 EXCL. EXCL. EXCL. EXCL. Certificate. "Weighing more than 110 kg., excluding 17 0103.92.02 those of codes 0103.92.01 and EXCL. -
• Lipids: a Heterogeneous Class of Naturally Occurring Organic
Organic Lecture Series 1 Organic Lecture Series •• Lipids:Lipids: a heterogeneous class of naturally occurring organic compounds classified together on the basis of common solubility properties –they are insoluble in water but soluble in aprotic organic solvents, including diethyl ether, methylene chloride, and acetone 2 Organic Lecture Series • Lipids include –triglycerides, phospholipids, prostaglandins, prostacyclins, and fat-soluble vitamins –cholesterol, steroid hormones, and bile acids 3 Organic Lecture Series TriglyceridesTriglycerides •• Triglyceride:Triglyceride: an ester of glycerol with three fatty acids O CH OCR O 2 CH 2 OH RCOOH 1. NaOH, H2 O R' COCH O HOCH + R'COOH 2 . HCl, H O 2 CH OH R' ' COOH CH 2 OCR'' 2 A triglyceride 1,2,3-Propanetriol Fatty acids (Glycerol, glycerin) 4 FattyFatty acidacid Organic Lecture Series • Fatty acid: a carboxylic acid derived from hydrolysis of animal fats, vegetable oils, or membrane phospholipids – nearly all have an even number of carbon atoms, most between 12 and 20, in an unbranched chain – the three most abundant are palmitic (16:0), stearic acid (18:0), and oleic acid (18:1) 5 FattyFatty acidacid Organic Lecture Series –the three most abundant are palmitic (16:0), stearic acid (18:0), and oleic acid (18:1) –In this labeling system: (carbon #:alkene #) palmitate (16:0) oleate (18:1) O O CH 2 OC(CH2 ) 14CH3 stearate (18:0) CH3 (CH2 )7 CH= CH( CH2 )7 COCH O CH 2 OC(CH2 ) 16CH3 6 FattyFatty acidacid Organic Lecture Series –in most unsaturated fatty acids, the cis isomer predominates; the -
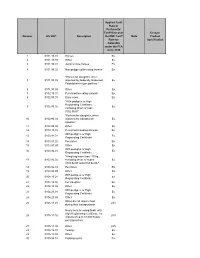
Number HS 2007 Description Applied Tariff Rate Or Preferential Tariff Rate
Applied Tariff Rate or Preferential Tariff Rate over Ex-out / Number HS 2007 Description the NMF Tariff Note Product Rate for Specification Colombia under the FTA since 2010 1 0101.10.01 Horses Ex. 2 0101.10.99 Other Ex. 3 0101.90.01 Jump or race horses Ex. 4 0101.90.02 Non pedigreed breeding horses Ex. "Horses for slaughter, when 5 0101.90.03 imported by Federally Inspected Ex. Establishment type packers." 6 0101.90.99 Other Ex. 7 0102.10.01 Pure-bred breeding animals Ex. 8 0102.90.01 Dairy cows Ex. "With pedigree or High Registrating Certificate, 9 0102.90.02 Ex. excluding those of code 0102.90.01" "Bovines for slaughter, when 10 0102.90.03 imported by Industrial de Ex. Abastos." 11 0102.90.99 Other Ex. 12 0103.10.01 Pure-bred breeding animals Ex. With pedigree or High 13 0103.91.01 Ex. Registrating Certificate. 14 0103.91.02 Peccaries Ex. 15 0103.91.99 Other Ex. With pedigree or High 16 0103.92.01 Ex. Registrating Certificate. "Weighing more than 110 kg., 17 0103.92.02 excluding those of codes Ex. 0103.92.01 and 0103.92.03." 18 0103.92.03 Peccaries Ex. 19 0103.92.99 Other Ex. With pedigree or High 20 0104.10.01 Ex. Registrating Certificate. 21 0104.10.02 For slaughter. Ex. 22 0104.10.99 Other Ex. With pedigree or High 23 0104.20.01 Ex. Registrating Certificate. 24 0104.20.99 Other Ex. When do not require food 25 0105.11.01 28% during their transportation Newly born breeding birds, with High Registrating Certificate, for 26 0105.11.02 28% imports of up to 18,000 heads per transaction. -

Phytochemical Investigation of Turraea Robusta, Turraea Nilotica and Ekebergia Capensis for Antiplasmodial and Cytotoxic Compounds
UNIVERSITY OF NAIROBI PHYTOCHEMICAL INVESTIGATION OF TURRAEA ROBUSTA, TURRAEA NILOTICA AND EKEBERGIA CAPENSIS FOR ANTIPLASMODIAL AND CYTOTOXIC COMPOUNDS. BY BEATRICE NJERI IRUNGU I80/80299/2010 A Thesis Submitted in Fulfilment of the Requirements for Award of the Degree of Doctor of Philosophy in Chemistry of the University of Nairobi 2014 i DECLARATION I declare tha t this thesis is my original work and has not been submitted elsewhere for examination or award of a degree. Signature.................................................... Date......................................... Beatrice Njeri Irungu I80/80299/2010 Department of Chemistry School of Physical Sciences University of Nairobi This thesis is submitted for examination with our approval as research supervisors Signature Date Professor Jacob Midiwo ................... ................. Department of Chemistry University of Nairobi P.O Box 30197-00100, Nairobi-Kenya [email protected] Professor Abiy Yenesew ……………… …………… Department of Chemistry University of Nairobi P.O Box 30197-00100, Nairobi-Kenya [email protected] Dr. Jennifer Orwa ........................ ........................ Kenya Medical Research Institute Centre for Traditional Medicine and Drug Research P.O. Box 54840-00200, Nairobi- Kenya [email protected] ii DEDICATION This thesis is dedicated to my husband Kimani Maina for enduring this long process with me, always offering support and love. our children Maina Kimani and Nyawira Kimani Though too young to understand what mum was going through, you supported me in your own little ways. I pray that this will serve as an encouragement as you walk through your academic pathways. iii ACKNOWLEDGEMENT The road towards a doctorate degree is not walked alone. Thus, this project would not have been possible without the support of my supervisors/mentors, friends and colleagues, to only some of whom it is possible to give particular mention here. -

WO 2012/148570 Al 1 November 2012 (01.11.2012) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2012/148570 Al 1 November 2012 (01.11.2012) P O P C T (51) International Patent Classification: AO, AT, AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, A61L 27/14 (2006.01) A61P 17/02 (2006.01) CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, C08L 101/16 (2006.01) DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, KR, (21) International Application Number: KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, PCT/US20 12/027464 MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, (22) International Filing Date: OM, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SC, SD, 2 March 2012 (02.03.2012) SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (25) Filing Language: English (84) Designated States (unless otherwise indicated, for every (26) Publication Language: English kind of regional protection available): ARIPO (BW, GH, (30) Priority Data: GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, SZ, TZ, 13/093,479 25 April 201 1 (25.04.201 1) US UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, MD, RU, TJ, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, (71) Applicant (for all designated States except US): DK, EE, ES, FI, FR, GB, GR, HR, HU, IE, IS, IT, LT, LU, WARSAW ORTHOPEDIC, INC. -

Georgia State Forensic Drugs
Comprehensive Forensic FT-IR Collection Library Listing – 4,286 spectra This extensive library contains materials not only of forensic interest but also for general problem solving and identification of unknown substances in industry and academia. The wide range of items include drugs, clandestine lab chemicals, explosives, paints, fabrics, dyes, polymers, inorganic compounds, pigments, adhesives, and other common materials. The library consists of 4,286 spectra that were acquired from a wide range of laboratories involved in forensic investigations. The collection includes the following classes of compounds: • Drugs of abuse, scheduled materials • Pharmaceuticals, vitamins and excipients • Clandestine lab materials and intermediates • Solvents, organic chemicals and hazardous chemicals • Accelerants • Lubricants and natural oils • Explosives, pyrotechnics, primers, powders and boosters • Herbal and plant material and fibers • Automobile paint vehicles, pigments, primers and clear coats • Textiles, natural and man-made fibers, carpet materials • Paints, coatings, varnishes, oils • Dyes and stains • Polymers, monomers, copolymers, plasticizers and rubbers • Inorganics, pigments, minerals and clays • Tape, adhesives, sealants, glues, caulks and putties • Crystal test derivatives and intermediates • Household chemicals, cleaning agents, surfactants and pesticide All spectra were measured using micro or macro Diamond ATR, thin films on salt windows or KBr pellets at 4 cm-1 spectral resolution. Comprehensive Forensic FT-IR Collection Index -

Atraric Acid Exhibits Anti-Inflammatory Effect in Lipopolysaccharide
International Journal of Molecular Sciences Article Atraric Acid Exhibits Anti-Inflammatory Effect in Lipopolysaccharide-Stimulated RAW264.7 Cells and Mouse Models 1, 2, 2 1 1 Seul-Ki Mun y, Kyung-Yun Kang y, Ho-Yeol Jang , Yun-Ho Hwang , Seong-Gyeol Hong , Su-Jin Kim 1, Hyun-Wook Cho 3, Dong-Jo Chang 1 , Jae-Seoun Hur 4 and Sung-Tae Yee 1,* 1 Department of Pharmacy, Sunchon National University, 255 Jungang-Ro, Suncheon 549-742, Korea; [email protected] (S.-K.M.); [email protected] (Y.-H.H.); [email protected] (S.-G.H.); [email protected] (S.-J.K.); [email protected] (D.-J.C.) 2 Suncheon Research Center for National Medicines, Suncheon 549-742, Korea; [email protected] (K.-Y.K.); [email protected] (H.-Y.J.) 3 Department of Biology, Sunchon National University, Suncheon 549-742, Korea; [email protected] 4 Department of Environmental Education, Korea Lichen Research Institute, Sunchon National University, Suncheon 549-742, Korea; [email protected] * Correspondence: [email protected]; Tel.: +82-61-750-3752; Fax: +82-61-750-3708 These authors contributed equally to this work. y Received: 26 August 2020; Accepted: 22 September 2020; Published: 25 September 2020 Abstract: Lichens, composite organisms resulting from the symbiotic association between the fungi and algae, produce a variety of secondary metabolites that exhibit pharmacological activities. This study aimed to investigate the anti-inflammatory activities of the secondary metabolite atraric acid produced by Heterodermia hypoleuca. The results confirmed that atraric acid could regulate induced pro-inflammatory cytokine, nitric oxide, prostaglandin E2, induced nitric oxide synthase and cyclooxygenase-2 enzyme expression in lipopolysaccharide (LPS)-stimulated RAW264.7 cells. -

Testoquench for Women Professional Guide
TestoQuench forWomen.com Professional Guide TestoQuench™ for Women Professional Guide The primary functions of TestoQuench™ for Women is to decrease excessive testosterone production and to decrease the effects of excessive testosterone and other androgens on testosterone sensitive tissues in women. The formulation uses phytoantiandrogens, a class of phyto-compounds that decrease tissue sensitivity to androgens or decrease androgen activity, such as through the action of 5-alpha- reductase inhibition, which decreases conversion of testosterone to the more androgenic dihydrotestosterone. Other actions include decreasing testosterone production, or androgen receptor antagonists. TestoQuench™ for Women has a synergistic combination of specific herbs that control the effects of excessive testosterone and other androgens by a number of actions including: • Anti-androgenogenic herb that decrease the production of testosterone. • 5-alpha-reductase inhibiting herbs that decrease production of Dihydrotestosterone (DHT) • Androgen receptor antagonists – that protect cells from excessive testosterone by blocking receptors • Supporting feminization of tissues with herbs that have estrogenic properties • Promoting healthy production and function of estrogens and progesterone, the endogenous hormones that naturally control the actions of testosterone in women. • Supporting healthy blood sugar levels • Anti-inflammatory actions decrease inflammation caused by androgen sensitivity. • Supporting healthy brain function, memory, and cognition, and enhancing