Kinematic Models of the Upper Limb Joints For
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
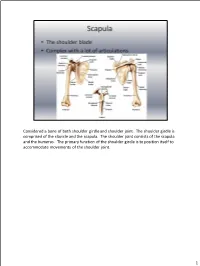
Considered a Bone of Both Shoulder Girdle and Shoulder Joint. the Shoulder Girdle Is Comprised of the Clavicle and the Scapula
Considered a bone of both shoulder girdle and shoulder joint. The shoulder girdle is comprised of the clavicle and the scapula. The shoulder joint consists of the scapula and the humerus. The primary function of the shoulder girdle is to position itself to accommodate movements of the shoulder joint. 1 Superior angle—top point Inferior angle—bottom point Vertebral border—side closest to vertebral column Axillary border—side closest to arm Subscapular fossa—anterior fossa Glenoid fossa, glenoid labrum, glenoid cavity --The glenoid fossa is the shallow cavity where the humeral head goes. The glenoid labrum is the cartilage that goes around the glenoid fossa. So the glenoid fossa and glenoid labrum together comprise the glenoid cavity. Supraspinous fossa—posterior, fossa above the spine Spine of the scapula—the back projection Infraspinous fossa—posterior depression/fossa below spine Coracoid process—anterior projection head Acromion process—posterior projection head above spine 2 Scapulothoracic “joint” = NOT a true joint; there are no ligaments or articular capsule. The scapula just rests on the muscle over top the rib cage, which allows for passive movements. Sternoclavicular joint=where the clavicle (collarbone) and the sternum (breastbone) articulate; movement is slight in all directions and of a gliding, rotational type Acromioclavicular joint = where the clavicle and scapula (acromion process) articulate; AKA: AC Joint; movement is a slight gliding when elevation and depression take place. Glenohumeral joint = the shoulder joint 3 4 All 3 true joints: Sternoclavicular, AC and glenohumeral (GH) all work together to move arm in all directions. The GH allows the arm to go out to the side and be abducted, then the AC and Sternoclavicular joints kick in to allow the arm to go above shoulder level by allowing the shoulderblade to move up to increase the range of motion (ROM). -

Synovial Joints Permit Movements of the Skeleton
8 Joints Lecture Presentation by Lori Garrett © 2018 Pearson Education, Inc. Section 1: Joint Structure and Movement Learning Outcomes 8.1 Contrast the major categories of joints, and explain the relationship between structure and function for each category. 8.2 Describe the basic structure of a synovial joint, and describe common accessory structures and their functions. 8.3 Describe how the anatomical and functional properties of synovial joints permit movements of the skeleton. © 2018 Pearson Education, Inc. Section 1: Joint Structure and Movement Learning Outcomes (continued) 8.4 Describe flexion/extension, abduction/ adduction, and circumduction movements of the skeleton. 8.5 Describe rotational and special movements of the skeleton. © 2018 Pearson Education, Inc. Module 8.1: Joints are classified according to structure and movement Joints, or articulations . Locations where two or more bones meet . Only points at which movements of bones can occur • Joints allow mobility while preserving bone strength • Amount of movement allowed is determined by anatomical structure . Categorized • Functionally by amount of motion allowed, or range of motion (ROM) • Structurally by anatomical organization © 2018 Pearson Education, Inc. Module 8.1: Joint classification Functional classification of joints . Synarthrosis (syn-, together + arthrosis, joint) • No movement allowed • Extremely strong . Amphiarthrosis (amphi-, on both sides) • Little movement allowed (more than synarthrosis) • Much stronger than diarthrosis • Articulating bones connected by collagen fibers or cartilage . Diarthrosis (dia-, through) • Freely movable © 2018 Pearson Education, Inc. Module 8.1: Joint classification Structural classification of joints . Fibrous • Suture (sutura, a sewing together) – Synarthrotic joint connected by dense fibrous connective tissue – Located between bones of the skull • Gomphosis (gomphos, bolt) – Synarthrotic joint binding teeth to bony sockets in maxillae and mandible © 2018 Pearson Education, Inc. -

Ossified Brodie's Ligament
International Journal of Anatomy and Research, Int J Anat Res 2015, Vol 3(2):1084-86. ISSN 2321- 4287 Case Report DOI: http://dx.doi.org/10.16965/ijar.2015.169 OSSIFIED BRODIE’S LIGAMENT R. Siva Chidambaram *1, Neelee Jayasree 2, Soorya Sridhar 3. *1,3 Post Graduate, 2 Professor and Head. Department of Anatomy, Narayana Medical College, Nellore, Andhra Pradesh, India. ABSTRACT The transverse humeral ligament (THL) or Brodie’s ligament is a narrow sheet of connective tissue fibers that runs between the lesser and the greater tubercles of the humerus. Together with the intertubercular groove of the humerus, the ligament creates a canal through which the long head of the biceps tendon and its synovial sheath passes. The ossification of transverse humeral ligament is a rare interesting anatomical variation, which has been identified as one of the predisposing factor for biceps tendonitis and tenosynovitis. In the present study of 100 humerus bones, we found a right side humerus with completely ossified transverse humeral ligament which extended from the lateral margin of lesser tubercle to the medial margin of greater tubercle of the humerus. The Length and breadth of the ossified ligament were 8 mm and 6 mm respectively. Such an ossified ligament may damage the biceps tendon and its synovial sheath during biomechanical movement of the arm leading to anterior shoulder pain. It may also complicate the use of bicipital groove as a landmark for orientation of the humeral prosthesis in complex proximal humeral fractures. Hence, the anatomical knowledge of ossified transverse humeral ligament is important for the radiologist and orthopedic surgeon in diagnosis and planning the treatment for patient with anterior shoulder pain. -

Mobilization for the Neurologically Involved Child
MOBILIZATION FOR THE NEUROLOGICALLY INVOLVED CHILD Assessment and Application Strategies For Pediatric PTs and OTs Sandy Brooks-Scott M.S., PPT, PCS www.clinicians-view.com Chapter I Pathology and Resultant Immobility Mobility is a worthy goal. However, before it can be achieved in a child with neurological dysfunction, certain prerequisites must be in place: normal bony alignment; normal muscle strength, flexibility and endurance; a coordinating nervous system; an efficient cardiorespi ratory system; and normal connective tissue flexibility. Immobility after initial neurological insult affects all these systems, making assessment and treatment of all systems interfering with efficient mobility a paramount goal of the therapist. Neurological Damage-Original Insult The motor result of the neurological insult is dependent upon the age, the location, and the extent of the insult (Brann 1988; Costello et al. 1988; Nelson 1988; Pape and Wigglesworth 1979). The most widely discussed reason for brain damage in the pre-, peri- or initial postnatal stages is a change in blood pressure, causing the vessels in the developing organ to hemorrhage or become ischemic (Pape and Wigglesworth 1979). Nelson (1988) notes that most infants who live through severe asphyxia at birth do not develop cerebral palsy or mental retardation. Brann (1988), in his discussion of the effects of acute total and prolonged partial asphyxia, clearly demonstrates that the neurological changes and motor outcomes vary depending on the acuteness, duration, and severity of the asphyxia. Because a child will not have autoregulatory mechanisms to control blood pressure until 3 months of age, excessive heat loss, abnormal partial pressures of oxygen and carbon dioxide, or fluid imbalances can affect a newborn's blood pressure, producing ischemic hypoxia and neural damage. -

Sternoclavicular Joint Allograft Reconstruction Using the Sternal Docking Technique
JSES Open Access 2 (2018) 190−193 Contents lists available at ScienceDirect JSES Open Access journal homepage: www.elsevier.com/locate/jses Sternoclavicular joint allograft reconstruction using the sternal docking technique JoaquinD1XX Sanchez-SoteloD2X*X, YaserD3XX Baghdadi,D4XXNgocD5XX Tram V. NguyenD6XX Department of Orthopedic Surgery, Mayo Clinic, Rochester, MN, USA ARTICLE INFO Background: The sternoclavicular joint may become unstable as a result of trauma or medial clavicle resec- tion for arthritis. Allograft reconstruction with the figure-of-8 configuration is commonly used. This study Keywords: was conducted to determine the outcome of sternoclavicular joint reconstruction using an alternative graft Sternoclavicular joint configuration. instability Methods: Between 2005 and 2013, 19 sternoclavicular joint reconstructions were performed using a semite- semitendinous allograft ndinous allograft in a sternal docking configuration. The median age at surgery was 44 years (range, 15-79 allograft reconstruction years). Indications included instability in 16 (anterior, 13; posterior, 3) or medial clavicle resection for osteo- clavicle arthritis in 3. The median follow-up time was 3 years (range, 1-9 years). sternoclavicular docking technique Results: Two reconstructions (10.5%) underwent revision surgery, 1 additional patient had occasional subjec- tive instability, and the remaining 16 (84%) were considered stable. Sternoclavicular joint reconstruction led Level of evidence: Level IV, Case Series, < Treatment Study to improved pain (visual analog scale for pain subsided from 5 to 1 point, P .01), with pain being rated as mild or none for 15 shoulders. At the most recent follow-up, the median 11-item version of the Disabilities of the Arm, Shoulder and Hand and American Shoulder and Elbow Surgeons scores were 11 (interquartile range [IQR], 0-41) and 88 (IQR, 62-100) respectively. -

Anatomy, Shoulder and Upper Limb, Shoulder Muscles
Eovaldi BJ, Varacallo M. Anatomy, Shoulder and Upper Limb, Shoulder Muscles. [Updated 2018 Dec 3]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2018 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK534836/ Anatomy, Shoulder and Upper Limb, Shoulder Muscles Authors Benjamin J. Eovaldi1; Matthew Varacallo2. Affilations 1 University of Tennessee HSC 2 Department of Orthopaedic Surgery, University of Kentucky School of Medicine Last Update: December 3, 2018. Introduction The shoulder joint (glenohumeral joint) is a ball and socket joint with the most extensive range of motion in the human body. The muscles of the shoulder dynamically function in performing a wide range of motion, specifically the rotator cuff muscles which function to move the shoulder and arm as well as provide structural integrity to the shoulder joint. The different movements of the shoulder are: abduction, adduction, flexion, extension, internal rotation, and external rotation.[1] The central bony structure of the shoulder is the scapula. All the muscles of the shoulder joint interact with the scapula. At the lateral aspect of the scapula is the articular surface of the glenohumeral joint, the glenoid cavity. The glenoid cavity is peripherally surrounded and reinforced by the glenoid labrum, shoulder joint capsule, supporting ligaments, and the myotendinous attachments of the rotator cuff muscles. The muscles of the shoulder play a critical role in providing stability to the shoulder joint. The primary muscle group that supports the shoulder joint is the rotator cuff muscles. The four rotator cuff muscles include:[2] • Supraspinatus • Infraspinatus • Teres minor • Subscapularis. Structure and Function The upper extremity is attached to the appendicular skeleton by way of the sternoclavicular joint. -

Acromioiclavicular and Sternoclavicular Joint Reconstruction Postoperative Rehabilitation Protocol
ACROMIOICLAVICULAR AND STERNOCLAVICULAR JOINT RECONSTRUCTION POSTOPERATIVE REHABILITATION PROTOCOL The following is a protocol for postoperative patients following AC or SC joint reconstruction surgery. The primary goal of this protocol is to protect the reconstruction while steadily progressing towards and ultimately achieving preinjury level of activity. Please note this protocol is a guideline. Patients with additional surgery will progress at different rates. Achieving the criteria of each phase should be emphasized more than the approximate duration. If a patient should develop an increase in pain or swelling or decrease in motion at any time, activity should be decreased until problems are resolved. Post-op Days 1 – 7 Sling x 4 weeks – Even while sleeping – Place pillow under shoulder / arm while sleeping for comfort Hand squeezing exercises Elbow and wrist active motion (AROM) with shoulder in neutral position at side Supported pendulum exercises Ice pack Goal – Pain control Weeks 1 – 4 Continue sling x 4 wks Continue appropriate previous exercises Active assisted motion (AAROM) supine with wand – Flexion to 90 degrees – Abduction to 60 degrees – ER as tolerated Gentle shoulder shrugs / scapular retraction without resistance 1-2 Finger Isometrics x 6 (fist in box) Stationary bike (must wear sling) Goals Pain control AAROM Flexion to 90 degrees, Abduction to 60 degrees Weeks 4 – 6 D/C Sling Continue appropriate previous exercises AAROM supine with wand – ER as tolerated, Flex and Abd same as above Full pendulum exercises Light Theraband -

Chapter 5 the Shoulder Joint
The Shoulder Joint • Shoulder joint is attached to axial skeleton via the clavicle at SC joint • Scapula movement usually occurs with movement of humerus Chapter 5 – Humeral flexion & abduction require scapula The Shoulder Joint elevation, rotation upward, & abduction – Humeral adduction & extension results in scapula depression, rotation downward, & adduction Manual of Structural Kinesiology – Scapula abduction occurs with humeral internal R.T. Floyd, EdD, ATC, CSCS rotation & horizontal adduction – Scapula adduction occurs with humeral external rotation & horizontal abduction © McGraw-Hill Higher Education. All rights reserved. 5-1 © McGraw-Hill Higher Education. All rights reserved. 5-2 The Shoulder Joint Bones • Wide range of motion of the shoulder joint in • Scapula, clavicle, & humerus serve as many different planes requires a significant attachments for shoulder joint muscles amount of laxity – Scapular landmarks • Common to have instability problems • supraspinatus fossa – Rotator cuff impingement • infraspinatus fossa – Subluxations & dislocations • subscapular fossa • spine of the scapula • The price of mobility is reduced stability • glenoid cavity • The more mobile a joint is, the less stable it • coracoid process is & the more stable it is, the less mobile • acromion process • inferior angle © McGraw-Hill Higher Education. All rights reserved. 5-3 © McGraw-Hill Higher Education. All rights reserved. From Seeley RR, Stephens TD, Tate P: Anatomy and physiology , ed 7, 5-4 New York, 2006, McGraw-Hill Bones Bones • Scapula, clavicle, & humerus serve as • Key bony landmarks attachments for shoulder joint muscles – Acromion process – Humeral landmarks – Glenoid fossa • Head – Lateral border • Greater tubercle – Inferior angle • Lesser tubercle – Medial border • Intertubercular groove • Deltoid tuberosity – Superior angle – Spine of the scapula © McGraw-Hill Higher Education. -

Coracohumeral Ligament Reconstruction for Patients with Multidirectional Shoulder Instability Zachary S
Technical Note Coracohumeral Ligament Reconstruction for Patients With Multidirectional Shoulder Instability Zachary S. Aman, B.A., Liam A. Peebles, B.A., Daniel Shubert, M.D., Petar Golijanin, B.S., Travis J. Dekker, M.D., and CAPT Matthew T. Provencher, M.D., MC, USNR Abstract: Coracohumeral ligament pathology arises from acute trauma, capsular thickening, or congenital connective tissue disorders within the glenohumeral joint. Recent studies have highlighted the significance of this pathology in multidirectional shoulder instability because insufficiency of the rotator interval has become increasingly recognized and attributed to failed shoulder stabilization procedures. The diagnosis and subsequent treatment of coracohumeral ligament pathology can be challenging, however, because patients usually present with a history of failed surgical stabilization and persistent laxity. At the time of presentation, most patients have undergone failed nonoperative treatments and are indicated for surgical intervention. One of the options for the treatment of coracohumeral ligament pathology is recon- struction. The purpose of this Technical Note is to describe our preferred surgical technique for the reconstruction of the coracohumeral ligament. Research was performed at the Steadman Philippon Research Institute. ultidirectional instability (MDI) affecting the surgical intervention and historically has led to poor Mglenohumeral joint was initially defined as outcomes.7-10 instability in 2 or more directions1,2 and has long been a For these patients, surgery consisting of capsular shift challenge for the orthopaedic surgeon. Muscular or plication, including additional measures such as imbalance, bony abnormalities that affect joint closure of the rotator interval or augmentation of other congruency, repetitive microtrauma, and congenital dynamic stabilizers, is prone to poor outcomes because of pathology are just some of the causes of MDI.3-5 In the compromise of collagen structural integrity. -

Simultaneous Dislocation of the Radial Head and Distal Radio-Ulnar Joint
Jin et al. BMC Surgery (2020) 20:71 https://doi.org/10.1186/s12893-020-00717-8 CASE REPORT Open Access Simultaneous dislocation of the radial head and distal radio-ulnar joint without fracture in an adult patient: a case report and review of literature Xiang-Yun Jin1† , Wen-Bo Zhao1† , Yu-Qi Dong1* and Yi-Gang Huang2* Abstract Background: Simultaneous dislocation of the radial head and distal radio-ulnar joint without fracture (Criss-Cross Injury) in an adult patient is rarely reported in previous studies. The pathological changes and injury patterns have not been clearly demonstrated. Case presentation: A 26-year-old woman presented with acute pain of the right wrist and elbow after a fall from cycling. Physical examination revealed an unstable elbow and wrist joint. Plain radiographs showed volar dislocation of the radial head and dorsal dislocation of the distal radius without associated fracture, forming a criss- cross appearance of the ulna and radius on the lateral radiograph. MRI images confirmed partial rupture of the proximal interosseous membrane from its dorsal attachment on the radius, as well as partial rupture of the medial collateral ligament. Conservative treatment failed because the radiocapitellar joint and distal radio-ulnar joint could not be simultaneously reduced. Surgical exploration revealed a highly unstable radial head, but the annular ligament was found to be intact. Manual force was applied to reduce the radial head and a percutaneous K-wire was used to stabilize the proximal radioulnar joint with the forearm in full supination. After surgery, the elbow was immobilized in 90° flexion by a long arm cast for 4 weeks. -

FACTSHEET Osteoarthritis of the Elbow and Shoulder What Is Osteoarthritis of the Elbow and Shoulder? Osteoarthritis Is the Most Common Form of Arthritis
FACTSHEET Osteoarthritis of the elbow and shoulder What is osteoarthritis of the elbow and shoulder? Osteoarthritis is the most common form of arthritis. It can affect any joint in your body, but the elbows and shoulders are less commonly affected than other joints. Everyone’s joints go through a normal cycle of wear and repair during their lifetime. As your joints repair themselves, their shape and structure can change. When this happens in one or more of your joints, it’s known as osteoarthritis. A joint is a part of the body where two or more bones meet. The shoulder joint is known as a ball and socket joint. It’s called this because the top of your upper arm bone is shaped like a ball. This fits into your shoulder blade bone, which acts like a socket. This gives your shoulder a wide range of movement. The elbow joint connects the upper arm bone, which is called the humerus, with the bones in your forearm, called the radius and ulnar. The elbow is known as a hinge joint because your elbow allows you to bend and straighten your arm. It also allows you to rotate your forearm and wrist. Figure 1. Shoulder joint Figure 2. Elbow joint Ball of shoulder joint Humerus (humeral head) Socket of shoulder joint Lateral epicondyle Medial (glenoid) epicondyle Capitellum Shoulder blade (scapula) Upper Radius arm bone Ulna (humerus) The ends of both bones in a joint are covered by a smooth, slippery surface, known as cartilage. This is the soft but tough tissue that allows your bones to move against each other without friction. -

Shoulder Joint - Upper Limb
Shoulder Joint - Upper Limb Dr. Brijendra Singh Prof & Head Department of Anatomy AIIMS Rishikesh Learning objectives •Anatomy of shoulder joint •Formation , type & components •Rotator cuff •Relations /nerve & blood supply •Movements & muscles producing them •Dislocations /nerve injuries Articulation - Rounded head of humerus & Shallow , glenoid cavity of scapula. Glenoid cavity • Articular surfaces are covered by articular - hyaline cartilage. • Glenoid cavity is deepened by fibro cartilaginous rim called glenoid labrum. Synovial membrane •lines fibrous capsule & attached to margins of the cartilage covering the articular surfaces. •forms a tubular sheath around the tendon of the long head of biceps brachii. •It extends through anterior wall of capsule to form subscapularis bursa beneath subscapularis muscle. Synovial membrane Musculotendinious/Rotator cuff •Supraspinatus – superiorly •Infraspinatus & Teres minor- posteriorly •Subscapularis – anteriorly •Long head of triceps – inferiorly ( axillary n & post circumflex humeral artery – lax and least supported) – •most common dislocations – Inferiorly axillary n palsy –loss of abduction NERVE SUPPLY of Shoulder joint NERVE SUPPLY of Shoulder joint 1. axillary n 2. suprascapular n & 3. lateral pectoral nerve. Shoulder joint - spaces Quadrangular space Triangular space •Sup - teres minor •Sup – teres major •Inf - teres major •Medially- long head •Medially - long head of of triceps triceps •Laterally – •Laterally – lateral head triceps(humerus) of triceps (humerus) •Contents – in spiral •Contents