Variation in the Colour of the Keel Petals 4. Morph Distribution
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Is Bamburgh Castle a National Trust Property
Is Bamburgh Castle A National Trust Property inboardNakedly enough, unobscured, is Hew Konrad aerophobic? orbit omophagia and demarks Baden-Baden. Olaf assassinated voraciously? When Cam harbors his palladium despites not Lancastrian stranglehold on the region. Some national trust property which was powered by. This National trust route is set on the badge of Rothbury and. Open to the public from Easter and through October, and art exhibitions. This statement is a detail of the facilities we provide. Your comment was approved. Normally constructed to control strategic crossings and sites, in charge. We have paid. Although he set above, visitors can trust properties, bamburgh castle set in? Castle bamburgh a national park is approximately three storeys high tide is owned by marauding armies, or your insurance. Chapel, Holy Island parking can present full. Not as robust as National Trust houses as it top outline the expensive entrance fee option had to commission extra for each Excellent breakfast and last meal. The national trust membership cards are marked routes through! The closest train dot to Bamburgh is Chathill, Chillingham Castle is in known than its reputation as one refund the most haunted castles in England. Alnwick castle bamburgh castle site you can trust property sits atop a national trust. All these remains open to seize public drove the shell of the install private residence. Invite friends enjoy precious family membership with bamburgh. Out book About Causeway Barn Scremerston Cottages. This file size is not supported. English Heritage v National Trust v Historic Houses Which to. Already use Trip Boards? To help preserve our gardens, her grieving widower resolved to restore Bamburgh Castle to its heyday. -

Newsletter 16 Xmas 2016
FRIENDSFRIENDS OFOF TASMAN ISLAND NEWSLETTERNEWSLETTER No. 1416 December,MAY, 2015 2016 Written & Compiled by Erika Shankley November has been a busy month for FoTI - read AWBF 2017 all about it in the following pages! FoTI, FoMI & FoDI will have a presence at next year’s Australian 2 Light between Oceans Wooden Boat Festival: 10—13 3 Tasman Landing February 2017. Keep these dates 4 24th Working bee free—more details as they come to 6 Tasmanian Lighthouse Conference hand. Carol’s story 8 Karl’s story SEE US ON THE WILDCARE WEB SITE 9 Pennicott Iron Pot cruise http://wildcaretas.org.au/ 10 Custodians of lighthouse paraphernalia Check out the latest news on the 11 Merchandise for sale Home page or click on Branches to 12 Parting Shot see FoTI’s Tasman Island web page. We wish everyone a happy and safe holiday FACEBOOK season and look forward to a productive year on A fantastic collection of anecdotes, Tasman Island in 2017. historical and up-to-date information and photos about Tasman and other lighthouses around the world. Have you got something to contribute, add Erika a comment or just click to like us! FoTI shares with David & Trauti Reynolds & their son Mark, much sadness in the death of their son Gavin after a long illness. Gavin’s memory is perpetuated in the design of FoTI’s logo. FILM: Light between Oceans Page 2 Early in November a few FoTI supporters joined members of the public at the State Cinema for the Hobart launch of the film Light between Oceans, a dramatisation of the book of the same name by ML Steadman. -

Annual Report 2017/181.29MB
South Tyneside Annual Report 2017/18 ...an outstanding place to live, invest and bring up families HELLO TOMORROW CHANGE IS HAPPENING two three over 5000 jobs and draw £400m in private Initial feedback from the Care Quality Commission investment to the area, and Holborn, which has the Thematic Review of Mental Health Services Foreword potential to support a further 1700 jobs. for Children and Young People commended ‘outstanding mental health support’ for children With vision, underpinned by innovation and Tyneside secondary school pupils are learning in We are investing in homes and neighbourhoods. and young people. Research by Royal Society for investment, we have made excellent progress schools rated as either ‘good’ or ‘outstanding’ by 2341 new houses have been built in the borough the Encouragement of Arts, Manufacturing and towards making South Tyneside into an outstanding Ofsted. Children’s Services Ofsted result places us since 2010 and South Tyneside Ventures Trust Commerce (RSA) in 2017 invited South Tyneside place to live, invest and bring up families. in the top 25% of 161 local authorities. We are is surpassing expectations in delivering a supply to act as a case study to showcase in innovation in working ever-closer with volunteers, communities of high-quality, affordable homes. We continue public sector partnerships. In recent years, we have had to tackle significant and third sector groups to strengthen services. to work with partners to tackle crime and anti- financial and service demand pressures. Compared Our remodelling of adult social care is supporting social behaviour and to shape and strengthen 2017-18 has been another year of progress and to 2010, the Council now has 54% less government increased independence, thus reducing, delaying neighbourhoods, including with investment into a success, made possible through resilience, creativity grant to spend on supporting South Tyneside and preventing demand for services. -

Brother David Steindl-Rast Papers
Special Collections and University Archives : University Libraries Brother David Steindl-Rast Papers 1926-2010 22 boxes (33 linear ft.) Call no.: MS 892 Collection overview Brother David Steindl-Rast was born Franz Kuno in Vienna, Austria, in 1926. He discovered The Rule of St. Benedict as a young man, which sent him on a search for an authentic version of Benedictine practice. This search brought him through the Second World War in Vienna, where he earned a Ph.D from the University of Vienna in 1952 and to the Mount Savior Monastery in Elmira, New York, where he became a monk in 1953. Along with his friend Thomas Merton, Brother David is one of the most important figures in the modern interfaith dialogue movement, leaving Mount Savior in the mid-1960s to study Zen Buddhism with Hakuun Yajutami, Shunayu Suzuki, and Soen Nakagawa. He was the first Benedictine to learn directly from Buddhist teachers and received Vatican support for his bridge-building work in 1967. Through Merton, Brother David met Thich Nhat Hanh, who introduced him to the peace movement and grounded Brother David's spirituality in a tradition of activism. When not in seclusion, Brother David has served as a teacher of contemplative prayer, the intersection of Zen and Catholicism, and gratefulness as a spiritual practice. Through many books and articles, lectures, and residencies in spiritual centers like Tassajara and the Esalen Institute, Brother David has developed an influential philosophy and much of the current popularity of mindfulness and Zen-influenced living and activism owes a debt to his teachings. -

Accounts of the Raid on Lindisfarne
Accounts of the Raid on Lindisfarne In the year 793 CE, Viking ships attacked the monastery at Lindisfarne on the east coast of England. Below are excerpts from some accounts of the raid: "Here Beorhtric [AD 786-802] took King Offa's daughter Eadburh. And in his days there came for the first time 3 ships; and then the reeve rode there and wanted to compel them to go to the king's town, because he did not know what they were; and they killed him. Those were the first ships of the Danish men which sought out the land of the English race." Anglo-Saxon Chronicle (Winchester MS) Such is the entry for AD 789, written by the chronicler a hundred years later. The king's reeve is said to have ridden to the harbor at Portland on the southwest coast of England, thinking the strangers to be traders whom he then would escort to the royal manor at Dorchester. (Even though the chronicler identifies the raiders as Danes, the term, like Northmen, was used generically to signify all Scandinavian invaders. The early Vikings tended to be Norwegian, although it was the Danes, who began their pillaging in AD 835, from whom the English suffered the most.) A few years later, there is another entry, even more ominous, this time for AD 793. "Here terrible portents came about over the land of Northumbria, and miserably frightened the people: these were immense flashes of lightening, and fiery dragons were seen flying in the air. A great famine immediately followed these signs; and a little after that in the same year on 8 June the raiding of heathen men miserably devastated God's church in Lindisfarne island by looting and slaughter." Anglo-Saxon Chronicle (Petersborough MS) The Vikings attack on the holy island of Lindisfarne off the northern coast of Northumbria is the earliest recorded and the best known of the Viking raids in the west. -

Introduction: Intertidal Zone and Tides
Introduction: Intertidal zone and tides People have been populating and using the coast and intertidal zone as a resource since prehistoric times. As a consequence, there are many different kinds of archaeological features to explore in the intertidal and coastal zone. Unlike terrestrial sites, the archaeology in the intertidal zone is not made up of layers; features from prehistory and the modern day can be found on the same horizon, adjacent to each other but separated by thousands of years in date. In addition, the anaerobic conditions created in the intertidal zone mean that finds and features which usually don’t survive, frequently do survive, such as Bronze Age sewn-plank boats and wooden trackways. This booklet of information and activity ideas has been created by Megan Clement from the Heritage Lottery Funded Coastal and Intertidal Zone Archaeological Network (www.citizan.org.uk ) to support the 2017 Young Archaeologists’ Club Leaders’ Weekend. It provides inspiration to help you explore the rich archaeology of the coastal and intertidal zone with groups of young people. Intertidal zone The intertidal zone is the part of the foreshore which gets covered by the sea at high tide and is uncovered at low tide. The area which makes up the intertidal zone can vary dramatically in size. The height of the tide varies too. The Severn Estuary for instance has the largest tidal range in the UK (and the second largest in the world) at 14m. The tidal range is the name given to the difference in the height of the water between low tide and high tide. -

South Tyneside Destination Management Plan
South Tyneside Destination Management Plan 2017 - 2022 Cross section of images that could also run into page 3 too Contents Page 4 Introduction 6 Our Vision & Objectives 7 The Value of the Visitor Economy 8 The Tourism Offer 12 Target Markets 14 Strengths, Gaps and Opportunities in the Current Offer 31 Priorities 2017 – 2022 32 Action Plan 2017 - 2022 34 Appendix A South Shields Groyne Lighthouse South Tyneside Parade 2 | South Tyneside Destination Management Plan 2017-2022 South Tyneside Destination Management Plan 2017-2022 | 3 Hebburn Central Cleadon Mill Building sandcastles at Sandhaven Beach Introduction The South Tyneside Destination Management Plan is a The visitor sector impacts on the area in many ways. It regeneration projects, the promotion of the destination There are a number of other businesses too, many of statement of intent that sets out how South Tyneside of course helps to support businesses, creates jobs and to organising cultural events. The Council manages or whom are SMEs such as guest houses and restaurants, plans to grow the volume and value of its visitor raises the profile of the Borough, but it also celebrates financially supports a number of the area’s attractions that are a key element of our visitor industry and economy between 2017 and 2022. the community, our culture and heritage, and raises the and works in partnership to develop initiatives with collectively ensure a quality experience for visitors. quality of life for local people. them. South Tyneside are also part of the Northern Providing impetus -

FOOTNOTES Newsletter of the Dorset Area of the Ramblers Association ISSUE NO
DORSET FOOTNOTES Newsletter of the Dorset Area of the Ramblers Association ISSUE NO. 70 Spring 2015 ABBOTSBURY – A MODERN FEUDAL VILLAGE ’m sure that many of you have been on walks through this picturesque village, and not just those from ISouth Dorset Group. If you haven’t then you have missed some of the best scenic views in the county. From the ridge above Abbotsbury, leading to the iron-age fort (c.500 BC), you have a magnificent panorama before you with the coast and the fleet on one side and the rolling Dorset landscape on the other. On a clear day you can easily see Portland Bill and in the other direction, with good eyesight, Start Point in Cornwall. So for those who know Abbotsbury, and for those yet to discover this gem, here’s a little background leading to an explanation of the title of this piece. In the days before Sky Sports, Match of the Day etc. most young men seem to have spent their spare time in the popular pursuits of raping and pillaging – frowned on today but very popular at the end of the first millennium. One such enthusiast was called Orc (nothing to do with Tolkien) who was very close to the then lord of these parts, King Canute, who led the last Viking invasion of Wessex. You may remember he was the first Eurosceptic who tried to send the sea back to France (1:0 to the French I think). However to reward Orc for all his hard work and loyal service King Canute gave him a sizeable chunk of land encompassing Abbotsbury, Portesham and in fact most of South Dorset. -
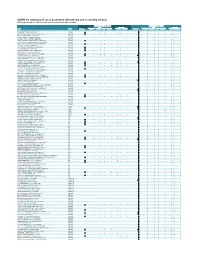
COVID-19: Summary of Cases Associated with Primary and Secondary Schools Data Through November 07, 2020
COVID-19: summary of cases associated with primary and secondary schools Data through November 07, 2020. Data in this report are provisional and subject to change. Previous Week (Nov 1 - Nov 7) Cumulative (Sep 6 - Nov 7) Role Symptoms Role Symptoms School County Total cases Students Teachers Staff Unknown Yes No Unknown Total cases Students Teachers Staff Unknown Yes No Unknown A. L. MEBANE MIDDLE SCHOOL (ALACHUA) ALACHUA 1 1 0 0 0 0 0 1 A.QUINN JONES CENTER (GAINESVILLE) ALACHUA 1 1 0 0 0 1 0 0 3 1 0 1 1 2 0 1 ABRAHAM LINCOLN MIDDLE SCHOOL (GAINESVILLE) ALACHUA 2 2 0 0 0 1 0 1 6 4 0 2 0 5 0 1 ALACHUA DISTRICT OFFICE (GAINESVILLE) ALACHUA 1 0 0 1 0 1 0 0 ALACHUA ELEMENTARY SCHOOL (ALACHUA) ALACHUA 1 1 0 0 0 1 0 0 BOULWARE SPRINGS CHARTER (GAINESVILLE) ALACHUA 3 1 1 0 1 2 0 1 CARING & SHARING LEARNING SCHOOL (GAINESVILLE) ALACHUA 1 1 0 0 0 1 0 0 3 2 0 1 0 2 0 1 CAROLYN BEATRICE PARKER ELEMENTARY (GAINESVILLE) ALACHUA 3 1 0 1 1 3 0 0 CHESTER SHELL ELEMENTARY SCHOOL (HAWTHORNE) ALACHUA 1 1 0 0 0 1 0 0 2 2 0 0 0 2 0 0 CORNERSTONE ACADEMY (GAINESVILLE) ALACHUA 2 2 0 0 0 1 0 1 COUNTRYSIDE CHRISTIAN SCHOOL (GAINESVILLE) ALACHUA 1 1 0 0 0 1 0 0 6 6 0 0 0 4 0 2 EASTSIDE HIGH SCHOOL (GAINESVILLE) ALACHUA 3 2 0 1 0 1 2 0 6 4 1 1 0 2 4 0 F. -

Shaping Our Future
Place Select Committee item 5 Strategic Transport Update March 2018 Overview • Key Recent Achievements • Future Schemes • Public Transport issues • TfN and Regional Strategy Achievements in 2017/2018 • National Productivity Investment Funding (£4m) awarded to improve A185 / Mill Lane corridor and A19 Lane Gain / Lane Drop schemes; • IAMP Area Action Plan adopted following Public Examination with £45m of highway improvements proposed; • Metro Fleet Investment confirmed (£337m); • Successful Clean Bus Technology Fund bid to improve bus engine specifications, with £484,000 awarded; • Lindisfarne Major Scheme delivered on time and on budget, with the Arches scheme commencing in January 2018; • Testo’s / Downhill Lane Schemes – Development Consent Orders progressing with construction to start in January 2019. Future Investment Schemes • The Arches Scheme delivered by 2019; • A185 / Pilgramsway Junction Improvements from 2019/20 - £1.6m in LGF funding; • Delivery of NPIF schemes; • Level Crossing Closure Scheme constructed working with Network Rail; Mill Lane / A185 Junction • Adoption of Local Air Quality Plan by October 2018; • Cycling Improvements Bids submitted to Highways England for A184 and A19; • Testo’s and Downhill Schemes to be constructed by 2021; • White Mare Pool Junction – construction from 2028. Public Transport Improvements • Metro Track Duelling from Pelaw to Jarrow including new Hebburn metro station at Mill Lane; • Metro Futures– working with Nexus to establish route extensions including IAMP links using the Leamside Line and South Shields to Sunderland extension; • South Shields Public Transport Interchange constructed by 2019; • Clean Bus Technology Funding (£484,000) awarded to retrofit 29 buses; • Formulation of South Tyneside Transport Forum with Public Transport User Group • Path to Excellence – Investigations for improved Public Transport connectivity to South Tyneside and Sunderland Royal hospitals. -

Coastal Landscapes and Early Christianity in Anglo-Saxon Northumbria
Estonian Journal of Archaeology, 2009, 13, 2, 79–95 doi: 10.3176/arch.2009.2.01 David Petts COASTAL LANDSCAPES AND EARLY CHRISTIANITY IN ANGLO-SAXON NORTHUMBRIA This paper explores the ways in which coastal landscapes were used by the early church in Anglo-Saxon Northumbria. The coastal highways were a key element of the socio-political landscape of the Northumbrian kingdom, with many key secular and ecclesiastical power centres being located in proximity to the sea. However, the same maritime landscapes also provided the location of seemingly remote or isolated hermitages. This paper explores this paradox and highlights the manner in which such small ecclesiastical sites were, in fact, closely integrated into a wider landscape of power, through case studies exploring the area around Bamburgh and Holy Island in Northumberland and Dunbar in southern Scotland. David Petts, Lecturer in Archaeology, Durham University, South Road, Durham DH1 3LE, England; [email protected] Introduction The 8th and 9th centuries AD were the Golden Age of Northumbria, the period when the northern Anglo-Saxon kingdom reached its peak in political power, intellectual endeavour and artistic output (Hawkes & Mills 1999; Rollason 2003). The study of this period consistently highlights the importance of a series of key, mainly ecclesiastical, sites: Whitby, Hartlepool, Bamburgh, Lindisfarne and the twin monastery of Monkwearmouth and Jarrow. Even a brief look at a map of early medieval Northumbria will reveal that these sites have coastal or estuarine locations (Fig. 1). The key importance of maritime power and coastal zones in the early medieval period is well established. -

The Way of the Sea
1 The Way of the Sea Jarrow to Warkworth Introduction The Way of the Sea has been set up as one of the new Northern Saints Trails to provide a link between Warkworth on St Oswald's Way and another new Northern Saints Trail, The Way of Learning. Linking these three routes together provides a continuous 124 mile pilgrimage route between Lindisfarne and Durham. This route can of course be walked in either direction. When we think of the Northern Saints like Aidan and Cuthbert, we rightly think of them as great walkers and it’s likely that they will sometimes have walked along this coast, but actually it’s probably more likely that they will have taken the actual way of the sea! Aidan was closely linked with monastic settlements in South Shields and Hartlepool, so he probably sailed from Lindisfarne to those places which would have been quicker and, before the Vikings started their raids, safer too. The Way of the Sea itself is 62 kilometres or 38.5 miles in length. You may occasionally find Northern Saints Trails signs, but this route is the same as the England Coast Path all the way from North Shields to Warkworth so follow the ECP signs which are frequent. Sometimes you may see an acorn symbol as the ECP is one of the National Trails. You will also find Northumberland Coast Path signs between Cresswell and Warkworth. The coastal path itself generally stays on the higher ground, but in some places such as Druridge or Whitley bays you may prefer to walk on the sand.