SKELETAL MICROSTRUCTURE INDICATES CHANCELLORIIDS and HALKIERIIDS ARE CLOSELY RELATED by SUSANNAH M
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Download Download
Dorjnamjaa et al. Mongolian Geoscientist 49 (2019) 41-49 https://doi.org/10.5564/mgs.v0i49.1226 Mongolian Geoscientist Review paper New scientific direction of the bacterial paleontology in Mongolia: an essence of investigation * Dorj Dorjnamjaa , Gundsambuu Altanshagai, Batkhuyag Enkhbaatar Department of Paleontology, Institute of Paleontology, Mongolian Academy of Sciences, Ulaanbaatar 15160, Mongolia *Corresponding author. Email: [email protected] ARTICLE INFO ABSTRACT Article history: We review the initial development of Bacterial Paleontology in Mongolia and Received 10 September 2019 present some electron microscopic images of fossil bacteria in different stages of Accepted 9 October 2019 preservation in sedimentary rocks. Indeed bacterial paleontology is one the youngest branches of paleontology. It has began in the end of 20th century and has developed rapidly in recent years. The main tasks of bacterial paleontology are detailed investigation of fossil microorganisms, in particular their morphology and sizes, conditions of burial and products of habitation that are reflected in lithological and geochemical features of rocks. Bacterial paleontology deals with fossil materials and is useful in analysis of the genesis of sedimentary rocks, and sedimentary mineral resources including oil and gas. The traditional paleontology is especially significant for evolution theory, biostratigraphy, biogeography and paleoecology; however bacterial paleontology is an essential first of all for sedimentology and for theories sedimentary ore genesis or biometallogeny Keywords: microfossils, phosphorite, sedimentary rocks, lagerstatten, biometallogeny INTRODUCTION all the microorganisms had lived and propagated Bacteria or microbes preserved well as fossils in without breakdowns. Bacterial paleontological various rocks, especially in sedimentary rocks data accompanied by the data on the first origin alike natural substances. -

The Cambrian Explosion: a Big Bang in the Evolution of Animals
The Cambrian Explosion A Big Bang in the Evolution of Animals Very suddenly, and at about the same horizon the world over, life showed up in the rocks with a bang. For most of Earth’s early history, there simply was no fossil record. Only recently have we come to discover otherwise: Life is virtually as old as the planet itself, and even the most ancient sedimentary rocks have yielded fossilized remains of primitive forms of life. NILES ELDREDGE, LIFE PULSE, EPISODES FROM THE STORY OF THE FOSSIL RECORD The Cambrian Explosion: A Big Bang in the Evolution of Animals Our home planet coalesced into a sphere about four-and-a-half-billion years ago, acquired water and carbon about four billion years ago, and less than a billion years later, according to microscopic fossils, organic cells began to show up in that inert matter. Single-celled life had begun. Single cells dominated life on the planet for billions of years before multicellular animals appeared. Fossils from 635,000 million years ago reveal fats that today are only produced by sponges. These biomarkers may be the earliest evidence of multi-cellular animals. Soon after we can see the shadowy impressions of more complex fans and jellies and things with no names that show that animal life was in an experimental phase (called the Ediacran period). Then suddenly, in the relatively short span of about twenty million years (given the usual pace of geologic time), life exploded in a radiation of abundance and diversity that contained the body plans of almost all the animals we know today. -

Dornbos.Web.CV
Stephen Quinn Dornbos Associate Professor and Department Chair Department of Geosciences University of Wisconsin-Milwaukee Milwaukee, WI 53201-0413 Phone: (414) 229-6630 Fax: (414) 229-5452 E-mail: [email protected] http://uwm.edu/geosciences/people/dornbos-stephen/ EDUCATION 2003 Ph.D., Geological Sciences, University of Southern California, Los Angeles, CA. 1999 M.S., Geological Sciences, University of Southern California, Los Angeles, CA. 1997 B.A., Geology, The College of Wooster, Wooster, OH. ADDITIONAL EDUCATION 2002 University of Washington, Summer Marine Invertebrate Zoology Course, Friday Harbor Laboratories. 1997 Louisiana State University, Summer Field Geology Course. PROFESSIONAL EXPERIENCE 2017-Present Department Chair, Department of Geosciences, University of Wisconsin-Milwaukee. 2010-Present Associate Professor, Department of Geosciences, University of Wisconsin-Milwaukee. 2004-2010 Assistant Professor, Department of Geosciences, University of Wisconsin-Milwaukee. 2012-Present Adjunct Curator, Geology Department, Milwaukee Public Museum. 2004-Present Curator, Greene Geological Museum, University of Wisconsin- Milwaukee. 2003-2004 Postdoctoral Research Fellow, Department of Earth Sciences, University of Southern California. 2002 Research Assistant, Invertebrate Paleontology Department, Natural History Museum of Los Angeles County. EDITORIAL POSITIONS 2017-Present Editorial Board, Heliyon. 2015-Present Board of Directors, Coquina Press. 2014-Present Commentaries Editor, Palaeontologia Electronica. 2006-Present Associate Editor, Palaeontologia Electronica. Curriculum Vitae – Stephen Q. Dornbos 2 RESEARCH INTERESTS 1) Evolution and preservation of early life on Earth. 2) Evolutionary paleoecology of early animals during the Cambrian radiation. 3) Geobiology of microbial structures in Precambrian–Cambrian sedimentary rocks. 4) Cambrian reef evolution, paleoecology, and extinction. 5) Exceptional fossil preservation. HONORS AND AWARDS 2013 UWM Authors Recognition Ceremony. 2011 Full Member, Sigma Xi. -

Geobiological Events in the Ediacaran Period
Geobiological Events in the Ediacaran Period Shuhai Xiao Department of Geosciences, Virginia Tech, Blacksburg, VA 24061, USA NSF; NASA; PRF; NSFC; Virginia Tech Geobiology Group; CAS; UNLV; UCR; ASU; UMD; Amherst; Subcommission of Neoproterozoic Stratigraphy; 1 Goals To review biological (e.g., acanthomorphic acritarchs; animals; rangeomorphs; biomineralizing animals), chemical (e.g., carbon and sulfur isotopes, oxygenation of deep oceans), and climatic (e.g., glaciations) events in the Ediacaran Period; To discuss integration and future directions in Ediacaran geobiology; 2 Knoll and Walter, 1992 • Acanthomorphic acritarchs in early and Ediacara fauna in late Ediacaran Period; • Strong carbon isotope variations; • Varanger-Laplandian glaciation; • What has happened since 1992? 3 Age Constraints: South China (538.2±1.5 Ma) 541 Ma Cambrian Dengying Ediacaran Sinian 551.1±0.7 Ma Doushantuo 632.5±0.5 Ma 635 Ma 635.2±0.6 Ma Nantuo (Tillite) 636 ± 5Ma Cryogenian Nanhuan 654 ± 4Ma Datangpo 663±4 Ma Neoproterozoic Neoproterozoic Jiangkou Group Banxi Group 725±10 Ma Tonian Qingbaikouan 1000 Ma • South China radiometric ages: Condon et al., 2005; Hoffmann et al., 2004; Zhou et al., 2004; Bowring et al., 2007; S. Zhang et al., 2008; Q. Zhang et al., 2008; • Additional ages from Nama Group (Namibia), Conception Group (Newfoundland), and Vendian (White Sea); 4 The Ediacaran Period Ediacara fossils Cambrian 545 Ma Nama assemblage 555 Ma White Sea assemblage 565 Ma Avalon assemblage 575 Ma 585 Ma Doushantuo biota 595 Ma 605 Ma Ediacaran Period 615 Ma -

Durham Research Online
Durham Research Online Deposited in DRO: 23 May 2017 Version of attached le: Accepted Version Peer-review status of attached le: Peer-reviewed Citation for published item: Betts, Marissa J. and Paterson, John R. and Jago, James B. and Jacquet, Sarah M. and Skovsted, Christian B. and Topper, Timothy P. and Brock, Glenn A. (2017) 'Global correlation of the early Cambrian of South Australia : shelly fauna of the Dailyatia odyssei Zone.', Gondwana research., 46 . pp. 240-279. Further information on publisher's website: https://doi.org/10.1016/j.gr.2017.02.007 Publisher's copyright statement: c 2017 This manuscript version is made available under the CC-BY-NC-ND 4.0 license http://creativecommons.org/licenses/by-nc-nd/4.0/ Additional information: Use policy The full-text may be used and/or reproduced, and given to third parties in any format or medium, without prior permission or charge, for personal research or study, educational, or not-for-prot purposes provided that: • a full bibliographic reference is made to the original source • a link is made to the metadata record in DRO • the full-text is not changed in any way The full-text must not be sold in any format or medium without the formal permission of the copyright holders. Please consult the full DRO policy for further details. Durham University Library, Stockton Road, Durham DH1 3LY, United Kingdom Tel : +44 (0)191 334 3042 | Fax : +44 (0)191 334 2971 https://dro.dur.ac.uk Accepted Manuscript Global correlation of the early Cambrian of South Australia: Shelly fauna of the Dailyatia odyssei Zone Marissa J. -

Contributions in BIOLOGY and GEOLOGY
MILWAUKEE PUBLIC MUSEUM Contributions In BIOLOGY and GEOLOGY Number 51 November 29, 1982 A Compendium of Fossil Marine Families J. John Sepkoski, Jr. MILWAUKEE PUBLIC MUSEUM Contributions in BIOLOGY and GEOLOGY Number 51 November 29, 1982 A COMPENDIUM OF FOSSIL MARINE FAMILIES J. JOHN SEPKOSKI, JR. Department of the Geophysical Sciences University of Chicago REVIEWERS FOR THIS PUBLICATION: Robert Gernant, University of Wisconsin-Milwaukee David M. Raup, Field Museum of Natural History Frederick R. Schram, San Diego Natural History Museum Peter M. Sheehan, Milwaukee Public Museum ISBN 0-893260-081-9 Milwaukee Public Museum Press Published by the Order of the Board of Trustees CONTENTS Abstract ---- ---------- -- - ----------------------- 2 Introduction -- --- -- ------ - - - ------- - ----------- - - - 2 Compendium ----------------------------- -- ------ 6 Protozoa ----- - ------- - - - -- -- - -------- - ------ - 6 Porifera------------- --- ---------------------- 9 Archaeocyatha -- - ------ - ------ - - -- ---------- - - - - 14 Coelenterata -- - -- --- -- - - -- - - - - -- - -- - -- - - -- -- - -- 17 Platyhelminthes - - -- - - - -- - - -- - -- - -- - -- -- --- - - - - - - 24 Rhynchocoela - ---- - - - - ---- --- ---- - - ----------- - 24 Priapulida ------ ---- - - - - -- - - -- - ------ - -- ------ 24 Nematoda - -- - --- --- -- - -- --- - -- --- ---- -- - - -- -- 24 Mollusca ------------- --- --------------- ------ 24 Sipunculida ---------- --- ------------ ---- -- --- - 46 Echiurida ------ - --- - - - - - --- --- - -- --- - -- - - --- -

Reinterpretation of the Enigmatic Ordovician Genus Bolboporites (Echinodermata)
Reinterpretation of the enigmatic Ordovician genus Bolboporites (Echinodermata). Emeric Gillet, Bertrand Lefebvre, Véronique Gardien, Emilie Steimetz, Christophe Durlet, Frédéric Marin To cite this version: Emeric Gillet, Bertrand Lefebvre, Véronique Gardien, Emilie Steimetz, Christophe Durlet, et al.. Reinterpretation of the enigmatic Ordovician genus Bolboporites (Echinodermata).. Zoosymposia, Magnolia Press, 2019, 15 (1), pp.44-70. 10.11646/zoosymposia.15.1.7. hal-02333918 HAL Id: hal-02333918 https://hal.archives-ouvertes.fr/hal-02333918 Submitted on 13 Nov 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. 1 Reinterpretation of the Enigmatic Ordovician Genus Bolboporites 2 (Echinodermata) 3 4 EMERIC GILLET1, BERTRAND LEFEBVRE1,3, VERONIQUE GARDIEN1, EMILIE 5 STEIMETZ2, CHRISTOPHE DURLET2 & FREDERIC MARIN2 6 7 1 Université de Lyon, UCBL, ENSL, CNRS, UMR 5276 LGL-TPE, 2 rue Raphaël Dubois, F- 8 69622 Villeurbanne, France 9 2 Université de Bourgogne - Franche Comté, CNRS, UMR 6282 Biogéosciences, 6 boulevard 10 Gabriel, F-2100 Dijon, France 11 3 Corresponding author, E-mail: [email protected] 12 13 Abstract 14 Bolboporites is an enigmatic Ordovician cone-shaped fossil, the precise nature and systematic affinities of 15 which have been controversial over almost two centuries. -

The Spence Shale Lagerstätte: an Important Window Into Cambrian Biodiversity
Downloaded from http://jgs.lyellcollection.org/ by guest on September 24, 2021 Accepted Manuscript Journal of the Geological Society The Spence Shale Lagerstätte: an Important Window into Cambrian Biodiversity Julien Kimmig, Luke C. Strotz, Sara R. Kimmig, Sven O. Egenhoff & Bruce S. Lieberman DOI: https://doi.org/10.1144/jgs2018-195 Received 31 October 2018 Revised 21 February 2019 Accepted 28 February 2019 © 2019 The Author(s). This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 License (http://creativecommons.org/licenses/by/4.0/). Published by The Geological Society of London. Publishing disclaimer: www.geolsoc.org.uk/pub_ethics Supplementary material at https://doi.org/10.6084/m9.figshare.c.4423145 To cite this article, please follow the guidance at http://www.geolsoc.org.uk/onlinefirst#cit_journal Downloaded from http://jgs.lyellcollection.org/ by guest on September 24, 2021 The Spence Shale Lagerstätte: an Important Window into Cambrian Biodiversity 1* 1,2 1,3 4 1,2 Julien Kimmig , Luke C. Strotz , Sara R. Kimmig , Sven O. Egenhoff & Bruce S. Lieberman 1Biodiversity Institute, University of Kansas, Lawrence, KS 66045, USA 2 Department of Ecology & Evolutionary Biology, University of Kansas, Lawrence, KS, USA 3Pacific Northwest National Laboratory, Richland, WA 99354, USA 4Department of Geosciences, Colorado State University, Fort Collins, CO 80523, USA *Correspondence: [email protected] Abstract: The Spence Shale Member of the Langston Formation is a Cambrian (Miaolingian: Wuliuan) Lagerstätte in northeastern Utah and southeastern Idaho. It is older than the more well- known Wheeler and Marjum Lagerstätten from western Utah, and the Burgess Shale from Canada. -
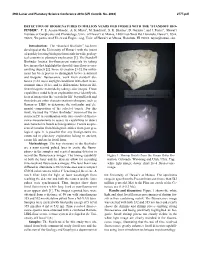
Detection of Biosignatures in Million Years Old Fossils with the “Standoff Bio- Finder”
49th Lunar and Planetary Science Conference 2018 (LPI Contrib. No. 2083) 2777.pdf DETECTION OF BIOSIGNATURES IN MILLION YEARS OLD FOSSILS WITH THE “STANDOFF BIO- FINDER”. T. E. Acosta-Maeda1, A. K. Misra1, M. Sandford1, S. K. Sharma1, D. Garmire2, and J. Porter1, 1Hawaiʻi Institute of Geophysics and Planetology, Univ. of Hawaiʻi at Mānoa, 1680 East-West Rd, Honolulu, Hawaiʻi, USA, 96822; 2Department of Electrical Engineering, Univ. of Hawaiʻi at Mānoa, Honolulu, HI 96822. [email protected]. Introduction: The “Standard Biofinder” has been developed at the University of Hawaiʻi with the intent of quickly locating biological materials in wide geolog- ical contexts in planetary exploration [1]. The Standoff Biofinder locates bio-fluorescent materials by taking live images that highlight the short lifetime fluorescence emitting objects [2]. Since its creation [3-5], the instru- ment has been proven to distinguish between mineral and biogenic fluorescence, work from standoff dis- tances (1-10 m) in daylight conditions with short meas- urement times (0.1s), and to differentiate between dif- ferent biogenic materials by taking color images. These capabilities could help an exploration rover identify ob- jects of interest for the ‘search for life’ beyond Earth and then dedicate other characterization techniques, such as Raman or LIBS, to determine the molecular and ele- mental composition of the selected targets. For this work, we used the “Color Biofinder” version of the in- strument [5] in combination with time-resolved fluores- cence measurements to assess its capabilities to detect and characterize fossils as biosignatures. Fossils are pre- served remains from biological entities from past geo- logical ages. -

Halwaxiids and the Early Evolution of the Lophotrochozoans. Science
REPORTS functions technique) of a quantum-mechanical n- and p-regions are equal (rh = re), charge 6. T. Ohta, A. Bostwick, T. Seyller, K. Horn, E. Rotenberg, analysis of oscillations of dj around the mi- carriers injected into graphene from the contact Science 313, 951 (2006). A-B 7. V. V. Cheianov, V. I. Fal’ko, Phys. Rev. B 74, 041403 rage image of a bilayer island formed on the S shown in Fig. 4A would meet again in the (2006). other side of symmetric PNJ in the monolayer focus at the distance 2w from the source 8. M. Katsnelson, K. Novoselov, A. Geim, Nat. Phys. 2, 620 sheet. To compare Fig. 2D shows the calculated (contact D3 in Fig. 4A). Varying the gate volt- (2006). mirage image of a spike of electrostatic potential age over the p-region changes the ratio n2 = 9. V. G. Veselago, Sov. Phys. Usp. 10, 509 (1968). r r 10. J. B. Pendry, Phys. Rev. Lett. 85, 3966 (2000). (smooth at the scale of the lattice constant in h/ e. This enables one to transform the focus 11. J. B. Pendry, Nature 423, 22 (2003). graphene), which induces LDOS oscillations into a cusp displaced by about 2(|n| −1)w along 12. D. R. Smith, J. B. Pendry, M. Wiltshire, Science 305, 788 equal on the two sublattices. The difference be- the x axis and, thus, to shift the strong coupling (2004). 13. M. S. Dresselhaus, G. Dresselhaus, Adv. Phys. 51, tween these two images is caused by the lack of from the pair of leads SD3 to either SD1 (for backscattering off A-B symmetric scatterers r < r )orSD (for r > r ). -

The Secrets of Fossils Lesson by Tucker Hirsch
The Secrets of Fossils Lesson by Tucker Hirsch Video Titles: Introduction: A New view of the Evolution of Animals Cambrian Explosion Jenny Clack, Paleontologist: The First Vertebrate Walks on Land Des Collins, Paleontologist: The Burgess Shale Activity Subject: Assessing evolutionary links NEXT GENERATION SCIENCE STANDARDS and evidence from comparative analysis of the fossil MS-LS4-1 Analyze and interpret data for patterns record and modern day organisms. in the fossil record that document the existence, diversity, extinction, and change of life forms Grade Level: 6 – 8 grades throughout the history of life on Earth under the Introduction assumptions that natural laws operate today as In this lesson students make connections between in the past. [Clarification Statement: Emphasis fossils and modern day organisms. Using the is on finding patterns of changes in the level of information about the Cambrian Explosion, they complexity of anatomical structures in organisms explore theories about how and why organisms diversified. Students hypothesize what evidence and the chronological order of fossil appearance might be helpful to connect fossil organisms to in the rock layers.] [Assessment Boundary: modern organisms to show evolutionary connections. Assessment does not include the names of Students use three videos from shapeoflife.org. individual species or geological eras in the fossil record.] Assessments Written MS-LS4-2 Apply scientific ideas to construct an Time 100-120 minutes (2 class periods) explanation for the anatomical similarities and Group Size Varies; single student, student pairs, differences among modern organisms and between entire class modern and fossil organisms to infer evolutionary relationships. [Clarification Statement: Emphasis Materials and Preparation is on explanations of the evolutionary relationships • Access to the Internet to watch 4 Shape of Life videos among organisms in terms of similarity or • Video Worksheet differences of the gross appearance of anatomical • “Ancient-Modern” Activity. -

Can Molecular Clocks and the Fossil Record Be Reconciled?
Prospects & Overviews Review essays The origin of animals: Can molecular clocks and the fossil record be reconciled? John A. Cunningham1)2)Ã, Alexander G. Liu1)†, Stefan Bengtson2) and Philip C. J. Donoghue1) The evolutionary emergence of animals is one of the most Introduction significant episodes in the history of life, but its timing remains poorly constrained. Molecular clocks estimate that The apparent absence of a fossil record prior to the appearance of trilobites in the Cambrian famously troubled Darwin. He animals originated and began diversifying over 100 million wrote in On the origin of species that if his theory of evolution years before the first definitive metazoan fossil evidence in were true “it is indisputable that before the lowest [Cambrian] the Cambrian. However, closer inspection reveals that clock stratum was deposited ... the world swarmed with living estimates and the fossil record are less divergent than is creatures.” Furthermore, he could give “no satisfactory answer” often claimed. Modern clock analyses do not predict the as to why older fossiliferous deposits had not been found [1]. In the intervening century and a half, a record of Precambrian presence of the crown-representatives of most animal phyla fossils has been discovered extending back over three billion in the Neoproterozoic. Furthermore, despite challenges years (popularly summarized in [2]). Nevertheless, “Darwin’s provided by incomplete preservation, a paucity of phylo- dilemma” regarding the origin and early evolution of Metazoa genetically informative characters, and uncertain expecta- arguably persists, because incontrovertible fossil evidence for tions of the anatomy of early animals, a number of animals remains largely, or some might say completely, absent Neoproterozoic fossils can reasonably be interpreted as from Neoproterozoic rocks [3].