Movement of Diuron and Hexazinone in Clay Soil and Infiltrated Pond Water
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2,4-Dichlorophenoxyacetic Acid
2,4-Dichlorophenoxyacetic acid 2,4-Dichlorophenoxyacetic acid IUPAC (2,4-dichlorophenoxy)acetic acid name 2,4-D Other hedonal names trinoxol Identifiers CAS [94-75-7] number SMILES OC(COC1=CC=C(Cl)C=C1Cl)=O ChemSpider 1441 ID Properties Molecular C H Cl O formula 8 6 2 3 Molar mass 221.04 g mol−1 Appearance white to yellow powder Melting point 140.5 °C (413.5 K) Boiling 160 °C (0.4 mm Hg) point Solubility in 900 mg/L (25 °C) water Related compounds Related 2,4,5-T, Dichlorprop compounds Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) 2,4-Dichlorophenoxyacetic acid (2,4-D) is a common systemic herbicide used in the control of broadleaf weeds. It is the most widely used herbicide in the world, and the third most commonly used in North America.[1] 2,4-D is also an important synthetic auxin, often used in laboratories for plant research and as a supplement in plant cell culture media such as MS medium. History 2,4-D was developed during World War II by a British team at Rothamsted Experimental Station, under the leadership of Judah Hirsch Quastel, aiming to increase crop yields for a nation at war.[citation needed] When it was commercially released in 1946, it became the first successful selective herbicide and allowed for greatly enhanced weed control in wheat, maize (corn), rice, and similar cereal grass crop, because it only kills dicots, leaving behind monocots. Mechanism of herbicide action 2,4-D is a synthetic auxin, which is a class of plant growth regulators. -

Herbicide Mode of Action Table High Resistance Risk
Herbicide Mode of Action Table High resistance risk Chemical family Active constituent (first registered trade name) GROUP 1 Inhibition of acetyl co-enzyme A carboxylase (ACC’ase inhibitors) clodinafop (Topik®), cyhalofop (Agixa®*, Barnstorm®), diclofop (Cheetah® Gold* Decision®*, Hoegrass®), Aryloxyphenoxy- fenoxaprop (Cheetah®, Gold*, Wildcat®), fluazifop propionates (FOPs) (Fusilade®), haloxyfop (Verdict®), propaquizafop (Shogun®), quizalofop (Targa®) Cyclohexanediones (DIMs) butroxydim (Factor®*), clethodim (Select®), profoxydim (Aura®), sethoxydim (Cheetah® Gold*, Decision®*), tralkoxydim (Achieve®) Phenylpyrazoles (DENs) pinoxaden (Axial®) GROUP 2 Inhibition of acetolactate synthase (ALS inhibitors), acetohydroxyacid synthase (AHAS) Imidazolinones (IMIs) imazamox (Intervix®*, Raptor®), imazapic (Bobcat I-Maxx®*, Flame®, Midas®*, OnDuty®*), imazapyr (Arsenal Xpress®*, Intervix®*, Lightning®*, Midas®* OnDuty®*), imazethapyr (Lightning®*, Spinnaker®) Pyrimidinyl–thio- bispyribac (Nominee®), pyrithiobac (Staple®) benzoates Sulfonylureas (SUs) azimsulfuron (Gulliver®), bensulfuron (Londax®), chlorsulfuron (Glean®), ethoxysulfuron (Hero®), foramsulfuron (Tribute®), halosulfuron (Sempra®), iodosulfuron (Hussar®), mesosulfuron (Atlantis®), metsulfuron (Ally®, Harmony®* M, Stinger®*, Trounce®*, Ultimate Brushweed®* Herbicide), prosulfuron (Casper®*), rimsulfuron (Titus®), sulfometuron (Oust®, Eucmix Pre Plant®*, Trimac Plus®*), sulfosulfuron (Monza®), thifensulfuron (Harmony®* M), triasulfuron (Logran®, Logran® B-Power®*), tribenuron (Express®), -

US EPA, Pesticide Product Label, TIDE USA HEXAZINONE 2SL,03/06
UNITED STATES ENVIRONMENTAL PROTECTION AGENCY WASHINGTON, DC 20460 OFFICE OF CHEMICAL SAFETY AND POLLUTION PREVENTION March 6, 2019 Ms. Katy DeGroot Regulatory Consultant Tide International, USA, Inc. c/o Pyxis Regulatory Consulting Inc. 4110 136th St. Ct. NW Gig Harbor, WA 98332 Subject: Notification per PRN 98-10 – Add optional referral statements. Product Name: Tide USA Hexazinone 2SL EPA Registration Number: 84229-35 Application Date: February 1, 2019 Decision Number: 548309 Dear Ms. DeGroot: The Agency is in receipt of your Application for Pesticide Notification under Pesticide Registration Notice (PRN) 98-10 for the above referenced product. The Registration Division (RD) has conducted a review of this request for its applicability under PRN 98-10 and finds that the action requested falls within the scope of PRN 98-10. The label submitted with the application has been stamped “Notification” and will be placed in our records. Should you wish to add/retain a reference to the company’s website on your label, then please be aware that the website becomes labeling under the Federal Insecticide Fungicide and Rodenticide Act and is subject to review by the Agency. If the website is false or misleading, the product would be misbranded and unlawful to sell or distribute under FIFRA section 12(a)(1)(E). 40 CFR 156.10(a)(5) list examples of statements EPA may consider false or misleading. In addition, regardless of whether a website is referenced on your product’s label, claims made on the website may not substantially differ from those claims approved through the registration process. Therefore, should the Agency find or if it is brought to our attention that a website contains false or misleading statements or claims substantially differing from the EPA approved registration, the website will be referred to the EPA’s Office of Enforcement and Compliance. -

Toxicology and Potential Health Risk of Chemicals That May Be Encountered by Workers Using Forest Vegetation Management Options
Toxicology and Potential Health Risk of Chemicals that May Be Encountered by Workers Using Forest Vegetation Management Options PART V: RISKS TO WORKERS USING HEXAZINONE FORMULATIONS (PRONONE® AND VELPAR®) Title Number Forest Practices Branch BC Ministry of Forests 7 Abstract Hexazinone is a broad-spectrum soil active herbicide used for site preparation, conifer release and in nurseries. The acute toxicity of hexazinone is low. Dermal toxicity is also very low, indicating poor absorption across the skin. It is irritant to the eyes, but does not produce skin sensitization. In a standard 90-day subchronic assay, rats consuming a diet containing 5000 ppm hexazinone (about 250 mg/kg/day) were unaffected except for slightly decreased weight gain. There was no effect at 1000 ppm. Dogs given 200 mg hexazinone/kg/day were unaffected except for modest weight loss. Two-year cancer studies of rats and mice indicated no detectable carcinogenic response, and there were no pathological changes other than benign adenomas (harmless tumours) found in the livers of mice maintained on a diet containing 10,000 ppm hexazinone. Lifetime systemic no-effect levels were 10 mg/kg/day for rats and 35 mg/kg/day for mice. Some liver effects were detectable at higher dose rates. Hexazinone was found to have no effect on reproduction and did not cause birth defects at doses that can be tolerated by the dams. It was shown to have limited mutagenic potential. Risks associated with use of hexazinone in forestry are slight, limited to eye and skin irritation. If daily intake is on the order of 0.03 mg/kg, which is to be expected of a worker who is moderately careful, the safety factor based on the no-observable-effect level (NOEL) of 10 mg/kg/day will be over 300. -

Risks of Linuron Use to Federally Threatened California Red-Legged Frog (Rana Aurora Draytonii)
Risks of Linuron Use to Federally Threatened California Red-legged Frog (Rana aurora draytonii) Pesticide Effects Determination Environmental Fate and Effects Division Office of Pesticide Programs Washington, D.C. 20460 June 19, 2008 Primary Authors: Michael Davy, Agronomist Wm. J. Shaughnessy, Ph.D, Environmental Scientist Environmental Risk Branch II Environmental Fate and Effects Division (7507C) Secondary Review: Donna Randall, Senior Effects Scientist Nelson Thurman, Senior Fate Scientist Environmental Risk Branch II Environmental Fate and Effects Division (7507P) Branch Chief, Environmental Risk Assessment Branch #: Arthur-Jean B. Williams, Acting Branch Chief Environmental Risk Branch II Environmental Fate and Effects Division (7507P) 2 Table of Contents 1. Executive Summary.................................................................................................8 2. Problem Formulation .............................................................................................14 2.1 Purpose...........................................................................................................................14 2.2 Scope..............................................................................................................................16 2.3 Previous Assessments ....................................................................................................18 2.4 Stressor Source and Distribution ...................................................................................19 2.4.1 Environmental Fate -

INDEX to PESTICIDE TYPES and FAMILIES and PART 180 TOLERANCE INFORMATION of PESTICIDE CHEMICALS in FOOD and FEED COMMODITIES
US Environmental Protection Agency Office of Pesticide Programs INDEX to PESTICIDE TYPES and FAMILIES and PART 180 TOLERANCE INFORMATION of PESTICIDE CHEMICALS in FOOD and FEED COMMODITIES Note: Pesticide tolerance information is updated in the Code of Federal Regulations on a weekly basis. EPA plans to update these indexes biannually. These indexes are current as of the date indicated in the pdf file. For the latest information on pesticide tolerances, please check the electronic Code of Federal Regulations (eCFR) at http://www.access.gpo.gov/nara/cfr/waisidx_07/40cfrv23_07.html 1 40 CFR Type Family Common name CAS Number PC code 180.163 Acaricide bridged diphenyl Dicofol (1,1-Bis(chlorophenyl)-2,2,2-trichloroethanol) 115-32-2 10501 180.198 Acaricide phosphonate Trichlorfon 52-68-6 57901 180.259 Acaricide sulfite ester Propargite 2312-35-8 97601 180.446 Acaricide tetrazine Clofentezine 74115-24-5 125501 180.448 Acaricide thiazolidine Hexythiazox 78587-05-0 128849 180.517 Acaricide phenylpyrazole Fipronil 120068-37-3 129121 180.566 Acaricide pyrazole Fenpyroximate 134098-61-6 129131 180.572 Acaricide carbazate Bifenazate 149877-41-8 586 180.593 Acaricide unclassified Etoxazole 153233-91-1 107091 180.599 Acaricide unclassified Acequinocyl 57960-19-7 6329 180.341 Acaricide, fungicide dinitrophenol Dinocap (2, 4-Dinitro-6-octylphenyl crotonate and 2,6-dinitro-4- 39300-45-3 36001 octylphenyl crotonate} 180.111 Acaricide, insecticide organophosphorus Malathion 121-75-5 57701 180.182 Acaricide, insecticide cyclodiene Endosulfan 115-29-7 79401 -

Control of Melaleuca Seedlings and Trees by Herbicides RANDALL K
J. Aquat. Plant Manage. 35: 55-59 Control of Melaleuca Seedlings and Trees by Herbicides RANDALL K. STOCKER1 AND D. R. SANDERS, SR.2 ABSTRACT starting in 1940 to prevent storm generated waves from erod- ing the levee system (Herbert Hoover Dike). From the tree Field tests of several herbicides at Lake Okeechobee, Flor- islands, melaleuca has spread into shallow wetland areas of ida, demonstrated effective control of melaleuca seedlings the lake. Because of the invasive nature of melaleuca (Craig- and mature trees. The lowest tested rates (4.5, 2.2, and 4.5 kg head 1971), and subsequent impacts to native plant (Myers ai/ha) of bromacil, hexazinone, and tebuthiuron (respec- 1984) and animal (Deuver et al. 1979, Maffei 1994, Mmazzot- tively) produced complete mortality of melaleuca seedings tii et al. 1981, Ostrenko et al 1979, Sowder and Woodall within six weeks of treatment. The highest tested rate (13.4 1985) communities, and because the USAE has determined kg ai/ha) of glyphosate also produced 100 percent mortality that the trees are no longer essential for bank stabilization, of seedlings, but 44 weeks were required to achieve these efforts have been underway since 1993 to control the trees results. At lower rates, tebuthiuron pellets were not as effec- and prevent further spread into adjacent wetlands. tive as the wettable powder formulation on seedlings. Only Melaleuca has been reported to be controlled by several dicamba + 2,4-D produced less than 100 percent mortality of herbicides. Imazapyr caused melaleuca mortality 12 months seedlings at the highest tested rate (87%). After 15 months, after treatment (Standish and Burns 1994). -

Transfer of Hexazinone and Glyphosate Through Undisturbed Soil Columns in Soils Under Christmas Tree Cultivation
Chemosphere 57 (2004) 265–272 www.elsevier.com/locate/chemosphere Transfer of hexazinone and glyphosate through undisturbed soil columns in soils under Christmas tree cultivation S. Dousset a,*, C. Chauvin a, P. Durlet b,M.Thevenot a a UMR 1229 INRA/Universite de Bourgogne, Geosol, Centre des Sciences de la Terre, Universite de Bourgogne, 6 boulevard Gabriel, 21000 Dijon, France b Parc Naturel Regional du Morvan, Maison du Parc, 58230 Saint Brisson, France Received 24 February 2004 Abstract Field studies monitoring pesticide pollution in the Morvan region (France) have revealed surface water contami- nation by some herbicides. The purpose of this study was to investigate in greater detail the transport of two herbicides, used in Christmas tree production in the Morvan, under controlled laboratory conditions. Thus, the leaching of hexazinone (3-cyclohexyl-6-dimethyl-amino-1-methyl-1,3,5-triazine-2,4 (1H,3H) dione) and glyphosate (N-(phosphono-methyl-glycine)) through structured soil columns was studied using one loamy sand and two sandy loams from sites currently under Christmas tree cultivation in the Morvan. The three soils were cultivated sandy brunisol [Sound reference base for soils, D. Baize, M.C. Girard (Coord.), INRA, Versailles, 1998, 322 p] or, according to the FAO [FAO, World reference base for soil resources, ISSS-ISRIC-FAO, FAO, Rome, Italy, 1998], the La Garenne was an arenosol and the two other soils were cambisols. The clay contents of the soils ranged from 86 to 156 gkgÀ1 and the organic carbon ranged from 98 to 347 g kgÀ1. After 160 mm of simulated rainfall applied over 12 days, 2– 11% of the applied hexazinone was recovered in the leachate. -

Cascade Range
United States bepartment of Agriculture Herbicide Forest Service Pacific Northwest Forest and Range and Conifer Experiment Station Research Paper PNW-292 Options for Reforesting October, 1981 Upper Slopes in the Cascade Range Edward J. Dimock II ROCKY Mof 'NTATN STATION This publication reports research involving pesticides. It does not contain recommendations for their use, nor does it imply that the uses discussed have been registered. All uses of pesticides must be registered by appropriate State and/or Federal agencies before they can be recommended. CAUTION: Pesticides can be injurious to humans, domestic animals, desirable Author plants, and fish or other wildlife -- if they I~ m w ~llmml are not handled or applied properly. Use EDWARD J. DIMOCK II is principal all pesticides selectively and carefully. silviculturist, Forestry Sciences Follow recommended practices for the Laboratory, 3200 Jefferson Way, disposal of surplus pesticides and Corvallis, Oregon 97331. pesticide containers. Abstract Summary Dimock, Edward J. I1. Herbicide and Nine herbicides (asulam, atrazine, Similar survival increases were also conifer options for reforesting upper bromacil, cyanazine, dalapon, consistently achieved with atrazine + slopes in the Cascade Range. Res. glyphosate, hexazinone, pronamide, and dalapon mixtures. Most successful Pap. PNW-292. Portland, OR: U.S. terbacil) were evaluated for control of applications of mixture produced 3-year Department of Agriculture, Forest sedge (Carex spp.) and beargrass survival of 62, 77, and 14 percent for white Service, Pacific Northwest Forest (Xerophyllum tenax [Pursh] Nutt.) to aid pine at the same three localities above. and Range Experiment Station; establishment of newly planted seedlings Survival of other conifers resulting from 1981.14 p. -

List of Herbicide Groups
List of herbicides Group Scientific name Trade name clodinafop (Topik®), cyhalofop (Barnstorm®), diclofop (Cheetah® Gold*, Decision®*, Hoegrass®), fenoxaprop (Cheetah® Gold* , Wildcat®), A Aryloxyphenoxypropionates fluazifop (Fusilade®, Fusion®*), haloxyfop (Verdict®), propaquizafop (Shogun®), quizalofop (Targa®) butroxydim (Falcon®, Fusion®*), clethodim (Select®), profoxydim A Cyclohexanediones (Aura®), sethoxydim (Cheetah® Gold*, Decision®*), tralkoxydim (Achieve®) A Phenylpyrazoles pinoxaden (Axial®) azimsulfuron (Gulliver®), bensulfuron (Londax®), chlorsulfuron (Glean®), ethoxysulfuron (Hero®), foramsulfuron (Tribute®), halosulfuron (Sempra®), iodosulfuron (Hussar®), mesosulfuron (Atlantis®), metsulfuron (Ally®, Harmony®* M, Stinger®*, Trounce®*, B Sulfonylureas Ultimate Brushweed®* Herbicide), prosulfuron (Casper®*), rimsulfuron (Titus®), sulfometuron (Oust®, Eucmix Pre Plant®*), sulfosulfuron (Monza®), thifensulfuron (Harmony®* M), triasulfuron, (Logran®, Logran® B Power®*), tribenuron (Express®), trifloxysulfuron (Envoke®, Krismat®*) florasulam (Paradigm®*, Vortex®*, X-Pand®*), flumetsulam B Triazolopyrimidines (Broadstrike®), metosulam (Eclipse®), pyroxsulam (Crusader®Rexade®*) imazamox (Intervix®*, Raptor®,), imazapic (Bobcat I-Maxx®*, Flame®, Midas®*, OnDuty®*), imazapyr (Arsenal Xpress®*, Intervix®*, B Imidazolinones Lightning®*, Midas®*, OnDuty®*), imazethapyr (Lightning®*, Spinnaker®) B Pyrimidinylthiobenzoates bispyribac (Nominee®), pyrithiobac (Staple®) C Amides: propanil (Stam®) C Benzothiadiazinones: bentazone (Basagran®, -
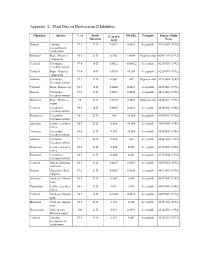
Appendix L Plant Data on Photosystem II Inhibitors
Appendix L Plant Data on Photosystem II Inhibitors Chemical Species % ai Study NOAEL Category Source (Study IC25 (Lb Duration Ai/A) Year) Diuron Tomato, 97.3 21 D 0.0017 0.0010 Acceptable 44113401 (1996) Lycopersicon esculentum Bromacil Rape, Brassica 95.9 21 D 0.002 <.0058 Supplemental 42491101 (1992) compestris Terbacil Cucumber, 97.4 14 D 0.0022 <0.00022 Acceptable 42336701 (1992) Cucumis sativus Terbacil Rape, Brassica 97.4 14 D 0.0035 <0.035 Acceptable 42336701 (1992) compestris Atrazine Cucumber, 97.7 21 D 0.004 .002 Supplemental 41223003 (1989) Cucumis sativus Terbacil Rape, Brassica sp. 96.9 14 D 0.0048 0.0031 Acceptable 43895801 (1996) Diuron Cucumber, 97.3 21 D 0.0053 0.0050 Acceptable 44113401 (1996) Cucumis sativus Bromacil Rape, Brassica 98 21 D 0.0055 0.0030 Supplemental 44488307 (1998) napus Terbacil Cucumber, 96.9 14 D 0.0058 0.0031 Acceptable 43895801 (1996) Cucumis sativus Prometryn Cucumber, 98.1 21 D .006 <0.006 Acceptable 41035903 (1988) Cucumis sativus Ametryn Lettuce, Lactuca 96.8 21 D 0.006 <0.006 Acceptable 40995809 (1988) sativa Ametryn Cucumber, 96.8 21 D 0.007 <0.006 Acceptable 40995809 (1988) Cucumis sativus Atrazine Cucumber, 97.7 21 D 0.008 .005 Acceptable 42041402 (1989) Cucumis sativus Prometon Lettuce, Lactuca 98.5 21 D 0.008 0.005 Acceptable 41725303 (1990) sativa Prometon Cucumber, 98.5 21 D 0.008 0.005 Acceptable 41725304 (1990) Cucumis sativus Terbacil Wheat, Triticum 96.9 14 D 0.0087 0.0063 Acceptable 43895801 (1996) aestivum Diuron Sugarbeet, Beta 97.3 21 D 0.0087 0.0050 Acceptable 44113401 (1996) -

Recommended Classification of Pesticides by Hazard and Guidelines to Classification 2019 Theinternational Programme on Chemical Safety (IPCS) Was Established in 1980
The WHO Recommended Classi cation of Pesticides by Hazard and Guidelines to Classi cation 2019 cation Hazard of Pesticides by and Guidelines to Classi The WHO Recommended Classi The WHO Recommended Classi cation of Pesticides by Hazard and Guidelines to Classi cation 2019 The WHO Recommended Classification of Pesticides by Hazard and Guidelines to Classification 2019 TheInternational Programme on Chemical Safety (IPCS) was established in 1980. The overall objectives of the IPCS are to establish the scientific basis for assessment of the risk to human health and the environment from exposure to chemicals, through international peer review processes, as a prerequisite for the promotion of chemical safety, and to provide technical assistance in strengthening national capacities for the sound management of chemicals. This publication was developed in the IOMC context. The contents do not necessarily reflect the views or stated policies of individual IOMC Participating Organizations. The Inter-Organization Programme for the Sound Management of Chemicals (IOMC) was established in 1995 following recommendations made by the 1992 UN Conference on Environment and Development to strengthen cooperation and increase international coordination in the field of chemical safety. The Participating Organizations are: FAO, ILO, UNDP, UNEP, UNIDO, UNITAR, WHO, World Bank and OECD. The purpose of the IOMC is to promote coordination of the policies and activities pursued by the Participating Organizations, jointly or separately, to achieve the sound management of chemicals in relation to human health and the environment. WHO recommended classification of pesticides by hazard and guidelines to classification, 2019 edition ISBN 978-92-4-000566-2 (electronic version) ISBN 978-92-4-000567-9 (print version) ISSN 1684-1042 © World Health Organization 2020 Some rights reserved.