Appendix 1 Technical Note
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Richard G. Hewlett and Jack M. Holl. Atoms
ATOMS PEACE WAR Eisenhower and the Atomic Energy Commission Richard G. Hewlett and lack M. Roll With a Foreword by Richard S. Kirkendall and an Essay on Sources by Roger M. Anders University of California Press Berkeley Los Angeles London Published 1989 by the University of California Press Berkeley and Los Angeles, California University of California Press, Ltd. London, England Prepared by the Atomic Energy Commission; work made for hire. Library of Congress Cataloging-in-Publication Data Hewlett, Richard G. Atoms for peace and war, 1953-1961. (California studies in the history of science) Bibliography: p. Includes index. 1. Nuclear energy—United States—History. 2. U.S. Atomic Energy Commission—History. 3. Eisenhower, Dwight D. (Dwight David), 1890-1969. 4. United States—Politics and government-1953-1961. I. Holl, Jack M. II. Title. III. Series. QC792. 7. H48 1989 333.79'24'0973 88-29578 ISBN 0-520-06018-0 (alk. paper) Printed in the United States of America 1 2 3 4 5 6 7 8 9 CONTENTS List of Illustrations vii List of Figures and Tables ix Foreword by Richard S. Kirkendall xi Preface xix Acknowledgements xxvii 1. A Secret Mission 1 2. The Eisenhower Imprint 17 3. The President and the Bomb 34 4. The Oppenheimer Case 73 5. The Political Arena 113 6. Nuclear Weapons: A New Reality 144 7. Nuclear Power for the Marketplace 183 8. Atoms for Peace: Building American Policy 209 9. Pursuit of the Peaceful Atom 238 10. The Seeds of Anxiety 271 11. Safeguards, EURATOM, and the International Agency 305 12. -
Grappling with the Bomb: Britain's Pacific H-Bomb Tests
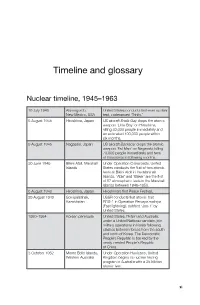
Timeline and glossary Nuclear timeline, 1945–1963 16 July 1945 Alamogordo, United States conducts first-ever nuclear New Mexico, USA test, codenamed ‘Trinity .’ 6 August 1945 Hiroshima, Japan US aircraft Enola Gay drops the atomic weapon ‘Little Boy’ on Hiroshima, killing 80,000 people immediately and an estimated 100,000 people within six months . 9 August 1945 Nagasaki, Japan US aircraft Bockscar drops the atomic weapon ‘Fat Man’ on Nagasaki, killing 70,000 people immediately and tens of thousands in following months . 30 June 1946 Bikini Atoll, Marshall Under Operation Crossroads, United Islands States conducts the first of two atomic tests at Bikini Atoll in the Marshall Islands. ‘Able’ and ‘Baker’ are the first of 67 atmospheric tests in the Marshall Islands between 1946–1958 . 6 August 1948 Hiroshima, Japan Hiroshima’s first Peace Festival. 29 August 1949 Semipalatinsk, USSR conducts first atomic test Kazakhstan RDS-1 in Operation Pervaya molniya (Fast lightning), dubbed ‘Joe-1’ by United States . 1950–1954 Korean peninsula United States, Britain and Australia, under a United Nations mandate, join military operations in Korea following clashes between forces from the south and north of Korea. The Democratic People’s Republic is backed by the newly created People’s Republic of China . 3 October 1952 Monte Bello Islands, Under Operation Hurricane, United Western Australia Kingdom begins its nuclear testing program in Australia with a 25 kiloton atomic test . xi GRAPPLING WITH THE BOMB 1 November 1952 Bikini Atoll, Marshall United States conducts its first Islands hydrogen bomb test, codenamed ‘Mike’ (10 .4 megatons) as part of Operation Ivy . -

The Los Alamos Thermonuclear Weapon Project, 1942-1952
Igniting The Light Elements: The Los Alamos Thermonuclear Weapon Project, 1942-1952 by Anne Fitzpatrick Dissertation submitted to the Faculty of Virginia Polytechnic Institute and State University in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY in SCIENCE AND TECHNOLOGY STUDIES Approved: Joseph C. Pitt, Chair Richard M. Burian Burton I. Kaufman Albert E. Moyer Richard Hirsh June 23, 1998 Blacksburg, Virginia Keywords: Nuclear Weapons, Computing, Physics, Los Alamos National Laboratory Igniting the Light Elements: The Los Alamos Thermonuclear Weapon Project, 1942-1952 by Anne Fitzpatrick Committee Chairman: Joseph C. Pitt Science and Technology Studies (ABSTRACT) The American system of nuclear weapons research and development was conceived and developed not as a result of technological determinism, but by a number of individual architects who promoted the growth of this large technologically-based complex. While some of the technological artifacts of this system, such as the fission weapons used in World War II, have been the subject of many historical studies, their technical successors -- fusion (or hydrogen) devices -- are representative of the largely unstudied highly secret realms of nuclear weapons science and engineering. In the postwar period a small number of Los Alamos Scientific Laboratory’s staff and affiliates were responsible for theoretical work on fusion weapons, yet the program was subject to both the provisions and constraints of the U. S. Atomic Energy Commission, of which Los Alamos was a part. The Commission leadership’s struggle to establish a mission for its network of laboratories, least of all to keep them operating, affected Los Alamos’s leaders’ decisions as to the course of weapons design and development projects. -

J. Robert Oppenheimer and His Colleagues Opposed Development of the Hydrogen Bomb
the years immediately following World War II, the United States was the only nation with the atomic bomb. Its strategic dominance, however, rested on a thin veneer of actual military capability. As late as 1947, the US did not have any atomic bombs assembled and ready for use. The Atomic Energy Commission, which held custody, was to work up the bombs and transfer them to the Air Force if and when they were needed. The Air Force had only a few airplanes, “Silver Plate” B-29s, that could deliver the bomb, and few trained crews. The leading atomic scientists who developed the atomic bomb during the war had left the Los Alamos weapons laboratory in New Mexico. Most of them were opposed to further military development of atomic energy. The US in 1946 proposed international control of atomic weapons. The offer to the United Nations fell through because the Soviets demanded the US eliminate its nuclear weapons as a precondition to agreement. J. Robert Oppenheimer and his colleagues opposed development of the hydrogen bomb. The fi rst thermonuclear explosion was the “Ivy Mike” test at Eniwetok Atoll in the Pacifi c in 1952. The fi reball was three miles wide and vaporized the coral islet on which the shot occurred. 62 AIR FORCE Magazine / October 2015 The concept for a far more powerful nuclear weapon—the hydrogen bomb, called the “Super” by the atomic scientists—had been around for some time. Few outside of the scientific community knew about it, and except for a few scattered advocates, there was almost no inter- est in pursuing it. -

Stalin and the Hydrogen Bomb 49 a Bomb Seemed Completely Unreal
48 The Short Millennium? Stalin and the The hydrogen bomb: the beginning of the project Hydrogen The atomic bomb was the artificial creation of Bomb man. Plutonium does not exist in nature, and uranium-235 does not accumulate anywhere in the quantities which produce the chain reaction of its almost instant fission. The idea of the hydrogen bomb was based on the physical phenomenon which is most widespread in the universe: nuclear synthesis, the formation of nuclear atoms of the heavier elements at the expense of the fusion of the nuclei of light elements. Almost all the elements of the earth’s Zhores A. Medvedev crust arose in this way. At high temperatures, of the order of hundreds of millions of degrees, the kinetic energy of the atoms of the light elements becomes so high that, colliding with themselves, they are able to ‘fuse’, forming In our last issue, in his nuclei of heavier elements. Hundreds of article entitled ‘Stalin and thousands times more energy is released at the the atomic bomb’, Zhores nuclear synthesis than at the fission of the Medvedev shed new light heavy atoms. Interest in the problem of nuclear on the beginnings of the fusion arose in the 1930s, especially after Hans nuclear era in the Soviet A. Bethe, the German physicist who, in 1934, Union. Now, he turns to the emigrated to the United States, developed the race to build the Soviet theory of the production of energy of the stars, Union’s thermonuclear or including the sun. According to this theory, hydrogen bomb. This article which was sufficiently well confirmed at the also contains much new time, the energy of the stars arose in the basic information. -

Cold War and Proliferation
Cold War and Proliferation After Trinity, Hiroshima, and Nagasaki and the defeat of Germany, the US believed to be in the absolute lead in nuclear weapon technology, US even supported Baruch plan for a short period of six months. But proliferation had started even before the Trinity test and developed rapidly to a whole set of Nuclear Powers over the following decades Spies and Proliferation At Potsdam conference 1945 Stalin was informed about US bomb project. Efficient Russian spy system in US had been established based on US communist cells and emigrant sympathies and worries about single dominant political and military power. Klaus Fuchs, German born British physicist, part of the British Collaboration at the Manhattan project passed information about Manhattan project and bomb development and design Plans to Russia. Arrested in 1949 in Britain and convicted to 14 years of prison. He served 9 years - returned to East Germany as Director of the Rossendorf Nuclear Research center. Fuchs case cause panic and enhanced security in US in times of cold war, fired by McCarthy propaganda. Numerous subsequent arrests and trials culminating in Rosenberg case! The second race for the bomb Start of soviet weapons program Program was ordered by Stalin in 1943 after being informed about US efforts. The administrative head of the program was Lavrenti Beria. Scientific director was Igor Kurchatov, Who headed the Russian nuclear research program and built the first Russian cyclotron in 1934. Lavrenty Pavlovich Beria (1899-1953), Soviet politician and police chief, is remembered chiefly as the executor of Stalin’s Great Purge of the 1930s His period of greatest power was during and after WW-II. -

Making the Russian Bomb from Stalin to Yeltsin
MAKING THE RUSSIAN BOMB FROM STALIN TO YELTSIN by Thomas B. Cochran Robert S. Norris and Oleg A. Bukharin A book by the Natural Resources Defense Council, Inc. Westview Press Boulder, San Francisco, Oxford Copyright Natural Resources Defense Council © 1995 Table of Contents List of Figures .................................................. List of Tables ................................................... Preface and Acknowledgements ..................................... CHAPTER ONE A BRIEF HISTORY OF THE SOVIET BOMB Russian and Soviet Nuclear Physics ............................... Towards the Atomic Bomb .......................................... Diverted by War ............................................. Full Speed Ahead ............................................ Establishment of the Test Site and the First Test ................ The Role of Espionage ............................................ Thermonuclear Weapons Developments ............................... Was Joe-4 a Hydrogen Bomb? .................................. Testing the Third Idea ...................................... Stalin's Death and the Reorganization of the Bomb Program ........ CHAPTER TWO AN OVERVIEW OF THE STOCKPILE AND COMPLEX The Nuclear Weapons Stockpile .................................... Ministry of Atomic Energy ........................................ The Nuclear Weapons Complex ...................................... Nuclear Weapon Design Laboratories ............................... Arzamas-16 .................................................. Chelyabinsk-70 -

Atmospheric Nuclear Weapons Testing
Battlefi eld of the Cold War The Nevada Test Site Volume I Atmospheric Nuclear Weapons Testing 1951 - 1963 United States Department of Energy Of related interest: Origins of the Nevada Test Site by Terrence R. Fehner and F. G. Gosling The Manhattan Project: Making the Atomic Bomb * by F. G. Gosling The United States Department of Energy: A Summary History, 1977 – 1994 * by Terrence R. Fehner and Jack M. Holl * Copies available from the U.S. Department of Energy 1000 Independence Ave. S.W., Washington, DC 20585 Attention: Offi ce of History and Heritage Resources Telephone: 301-903-5431 DOE/MA-0003 Terrence R. Fehner & F. G. Gosling Offi ce of History and Heritage Resources Executive Secretariat Offi ce of Management Department of Energy September 2006 Battlefi eld of the Cold War The Nevada Test Site Volume I Atmospheric Nuclear Weapons Testing 1951-1963 Volume II Underground Nuclear Weapons Testing 1957-1992 (projected) These volumes are a joint project of the Offi ce of History and Heritage Resources and the National Nuclear Security Administration. Acknowledgements Atmospheric Nuclear Weapons Testing, Volume I of Battlefi eld of the Cold War: The Nevada Test Site, was written in conjunction with the opening of the Atomic Testing Museum in Las Vegas, Nevada. The museum with its state-of-the-art facility is the culmination of a unique cooperative effort among cross-governmental, community, and private sector partners. The initial impetus was provided by the Nevada Test Site Historical Foundation, a group primarily consisting of former U.S. Department of Energy and Nevada Test Site federal and contractor employees. -

The Hydrogen Bomb
1 APRIL 2002 DRAFT: PLEASE DO NOT QUOTE OR CITE CHAPTER FOUR: THE HYDROGEN BOMB By David Holloway Origins It was the prospect of the uranium bomb that gave rise to the idea of the hydrogen bomb. In the years before World War II physicists had identified the nuclear fusion of light elements as the source of energy in the sun and the stars. Since fusion takes place only at temperatures of tens of millions of degrees, this research did not appear to have practical application.1 Early in 1942 Enrico Fermi speculated, in a conversation with Edward Teller, that a fission explosion could be used to initiate a thermonuclear reaction in a mass of deuterium, one of the isotopes of hydrogen. Fermi and Teller understood from the outset that the explosive yield of a fusion bomb could be made indefinitely large, depending only on the amount of thermonuclear fuel it contained. Teller went on to examine the idea of a thermonuclear bomb and in the summer of 1942 presented his preliminary ideas to the Berkeley conference on the physics of nuclear weapons. When Los Alamos was established in the spring of 1943, 2 work on the superbomb or Super (as the hydrogen bomb was known) was one of its main tasks.2 It soon became apparent, however, that the Super would be difficult to develop. Significant amounts of tritium – another, heavier, isotope of hydrogen – would be needed, but tritium occurs rarely in nature and is difficult and costly to produce. This lessened the promise of the hydrogen bomb as a wartime project. -

Chapter Six Conclusion: the Super, the System, and Its Critical Problems
Chapter Six Conclusion: The Super, The System, and Its Critical Problems A decade passed between the time Fermi first proposed the idea of a fusion bomb until the Mike test. Compared to the wartime fission weapon project, Los Alamos appeared to take a considerably longer time to complete research and development of an H-bomb. It is difficult to compare the two projects, however, because the weapons technologies differed from one another excessively, and so did the systems they were developed in. In addition, the Laboratory focused on the Classical Super configuration for the majority of the time, with greater seriousness directed at it than towards any other theory. Up until 1951, the Super represented almost the entire Los Alamos thermonuclear program, with the Alarm Clock and Booster as the only theoretical alternatives. The length of time the U.S. took to develop and test a viable hydrogen bomb, too, is problematic in that it has taken on the form of historical myth. I will elaborate on this later. The period of time that Los Alamos needed to develop and test a fusion device is relative historically. When comparing the time it took Los Alamos to develop a fission bomb as opposed to a fusion bomb it is necessary to consider the characteristics of each project, and the conditions surrounding their development: Compared with the gun and implosion bombs, a fusion weapon involved a much more complicated set of physical problems to solve, 287 fewer people participated in this work, no deadline had been set, and no military directive for this project existed. -

Soviet Hydrogen Bomb
The secret of the SOVIET HYDROGEN BOMB Alex Wellerstein and Edward Geist Was the first Soviet thermonuclear device really a step in the wrong direction? Alex Wellerstein ([email protected]) is an assistant professor of science and technology studies at Stevens Institute of Technology in Hoboken, New Jersey, and Edward Geist is a policy researcher at Rand Corp in Santa Monica, California. o bomb design has been as much maligned or absolutely crucial in helping to solve otherwise disparaged as the first Soviet thermonuclear the riddle of how the Soviets developed their later, multi-stage, multi-megaton weapon. Detonated in August 1953, the bomb, thermonuclear weapon designs. In- officially tested under the name RDS-6s but usually deed, the Sloika is possibly the answer N to the most curious question about the known as Sloika or “layer cake” (the name Andrei Soviet thermonuclear program: How Sakharov coined for it), was nothing to sneeze at. Shown in figure 1 did it develop a form of the Teller– and able to be dropped from aircraft, it released the explosive Ulam design only a year after develop- ing the Sloika?1 equivalent, or yield, of almost half a megaton of TNT. The result was a blazing fireball with 20 times the power of the bomb that leveled Why do we care? Nagasaki, Japan. The origins of the reflexive diminishing of the Sloika are found, in part, in an earlier debate in the US over whether But when discussed today, the Sloika is almost immediately to develop an H-bomb at all. In the fall of 1949, after the first qualified by US experts as not a “true” hydrogen bomb. -

Cold War and Proliferation
Cold War and Proliferation After Trinity, Hiroshima, and Nagasaki and the defeat of Germany, the US believed to be in the absolute lead in nuclear weapon technology, US even supported Baruch plan for a short period of six months. But proliferation had started even before the Trinity test and developed rapidly to a whole set of Nuclear Powers over the following decades Spies and Proliferation At Potsdam conference 1945 Stalin was informed about US bomb project. Efficient Russian spy system in US had been established based on US communist cells and emigrant sympathies and worries about single dominant political and military power. Klaus Fuchs, German born British physicist, part of the British Collaboration at the Manhattan project passed information about Manhattan project and bomb development and design Plans to Russia. Arrested in 1949 in Britain and convicted to 14 years of prison. He served 9 years - returned to East Germany as Director of the Rossendorf Nuclear Research center. Fuchs case caused panic and enhanced security in US. Fear of communist take-over was fired by McCarthy propaganda. Numerous subsequent arrests and trials culminating in Rosenberg case! The Spy Story Fat Man Design Copied by Klaus Fuchs and forwarded to Russian courier person (Harry Gold). Spy Scare Value of Spy Work, Information on trends not detail of design It is unknown whether Fuchs' fission information had a substantial impact. Considering that the pace of the Soviet program was set primarily by the amount of uranium they could procure, it is hard for scholars to accurately judge how much "time" this saved the Soviets.