The Effect of Insulin Dysregulation and Breed on HPA Axis Function and Plasma Cortisol Binding Dynamics in Ponies and Horses
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Multifaceted Physiological Roles of Adiponectin in Inflammation And
International Journal of Molecular Sciences Review Multifaceted Physiological Roles of Adiponectin in Inflammation and Diseases Hyung Muk Choi 1, Hari Madhuri Doss 1,2 and Kyoung Soo Kim 1,2,* 1 Department of Clinical Pharmacology and Therapeutics, Kyung Hee University School of Medicine, Seoul 02447, Korea; [email protected] (H.M.C.); [email protected] (H.M.D.) 2 East-West Bone & Joint Disease Research Institute, Kyung Hee University Hospital at Gangdong, Gandong-gu, Seoul 02447, Korea * Correspondence: [email protected]; Tel.: +82-2-961-9619 Received: 3 January 2020; Accepted: 10 February 2020; Published: 12 February 2020 Abstract: Adiponectin is the richest adipokine in human plasma, and it is mainly secreted from white adipose tissue. Adiponectin circulates in blood as high-molecular, middle-molecular, and low-molecular weight isoforms. Numerous studies have demonstrated its insulin-sensitizing, anti-atherogenic, and anti-inflammatory effects. Additionally, decreased serum levels of adiponectin is associated with chronic inflammation of metabolic disorders including Type 2 diabetes, obesity, and atherosclerosis. However, recent studies showed that adiponectin could have pro-inflammatory roles in patients with autoimmune diseases. In particular, its high serum level was positively associated with inflammation severity and pathological progression in rheumatoid arthritis, chronic kidney disease, and inflammatory bowel disease. Thus, adiponectin seems to have both pro-inflammatory and anti-inflammatory effects. This indirectly indicates that adiponectin has different physiological roles according to an isoform and effector tissue. Knowledge on the specific functions of isoforms would help develop potential anti-inflammatory therapeutics to target specific adiponectin isoforms against metabolic disorders and autoimmune diseases. -

Pharmacokinetics of Omarigliptin, a Once-Weekly Dipeptidyl Peptidase-4 Inhibitor
Available online a t www.derpharmachemica.com ISSN 0975-413X Der Pharma Chemica, 2016, 8(12):292-295 CODEN (USA): PCHHAX (http://derpharmachemica.com/archive.html) Mini-review: Pharmacokinetics of Omarigliptin, a Once-weekly Dipeptidyl Peptidase-4 Inhibitor Nermeen Ashoush a,b aClinical Pharmacy and Pharmacy Practice Department, Faculty of Pharmacy, British University in Egypt, El- Sherouk city, Cairo 11837, Egypt. bHead of Health Economics Unit, Center for Drug Research and Development (CDRD), Faculty of Pharmacy, British University in Egypt, El-Sherouk city, Cairo 11837, Egypt. _____________________________________________________________________________________________ ABSTRACT The dipeptidyl peptidase-4 (DPP-4) inhibitors are novel oral hypoglycemic drugs which have been in clinical use for the past 10 years. The drugs are safe, weight neutral and widely prescribed. There are currently many gliptins approved by FDA, namely sitagliptin, vildagliptin, saxagliptin, linagliptin, alogliptin with several more in advanced stages of development. The gliptins may possess cardiovascular protective effects and their administration may promote β-cell survival; claims currently being evaluated in clinical and preclinical studies. The gliptins are an optional second-line therapy after metformin; they are generally well tolerated with low risk of hypoglycemia. The various compounds differ with respect to their pharmacokinetic properties; however, their clinical efficacy appears to be similar. The clinical differences between the various compounds -

An in Vivo Investigation Into the Actions of the Hypothalamic Neuropeptide, QRFP
An In Vivo Investigation into the Actions of the Hypothalamic Neuropeptide, QRFP A thesis submitted to the University of Manchester for the degree of Doctor of Philosophy in the Faculty of Biology, Medicine and Health Christopher J Cook Faculty of Biology, Medicine and Health School of Medical Sciences 2017 Contents Abstract ........................................................................................................................................... 11 Declaration ........................................................................................................................................ 12 Copyright ........................................................................................................................................... 12 Acknowledgement ............................................................................................................................ 13 Chapter 1 Introduction ................................................................................................. 14 1.1 Energy homeostasis ................................................................................................................ 15 1.2 The control of food intake ...................................................................................................... 16 1.2.1 Peripheral signals regulating food intake .......................................................................... 17 1.2.2 Central aspects of food intake regulation ........................................................................ -

(Title of the Thesis)*
THE PHYSIOLOGICAL ACTIONS OF ADIPONECTIN IN CENTRAL AUTONOMIC NUCLEI: IMPLICATIONS FOR THE INTEGRATIVE CONTROL OF ENERGY HOMEOSTASIS by Ted Donald Hoyda A thesis submitted to the Department of Physiology In conformity with the requirements for the degree of Doctor of Philosophy Queen‟s University Kingston, Ontario, Canada (September, 2009) Copyright © Ted Donald Hoyda, 2009 ABSTRACT Adiponectin regulates feeding behavior, energy expenditure and autonomic function through the activation of two receptors present in nuclei throughout the central nervous system, however much remains unknown about the mechanisms mediating these effects. Here I investigate the actions of adiponectin in autonomic centers of the hypothalamus (the paraventricular nucleus) and brainstem (the nucleus of the solitary tract) through examining molecular, electrical, hormonal and physiological consequences of peptidergic signalling. RT-PCR and in situ hybridization experiments demonstrate the presence of AdipoR1 and AdipoR2 mRNA in the paraventricular nucleus. Investigation of the electrical consequences following receptor activation in the paraventricular nucleus indicates that magnocellular-oxytocin cells are homogeneously inhibited while magnocellular-vasopressin neurons display mixed responses. Single cell RT-PCR analysis shows oxytocin neurons express both receptors while vasopressin neurons express either both receptors or one receptor. Co-expressing oxytocin and vasopressin neurons express neither receptor and are not affected by adiponectin. Median eminence projecting corticotropin releasing hormone neurons, brainstem projecting oxytocin neurons, and thyrotropin releasing hormone neurons are all depolarized by adiponectin. Plasma adrenocorticotropin hormone concentration is increased following intracerebroventricular injections of adiponectin. I demonstrate that the nucleus of the solitary tract, the primary cardiovascular regulation site of the medulla, expresses mRNA for AdipoR1 and AdipoR2 and mediates adiponectin induced hypotension. -

Safety and Efficacy of Omarigliptin (MK-3102), a Novel Once-Weekly
2106 Diabetes Care Volume 38, November 2015 fi Wayne H.-H. Sheu,1 Ira Gantz,2 Safety and Ef cacy of Omarigliptin Menghui Chen,2 Shailaja Suryawanshi,2 Arpana Mirza,2 Barry J. Goldstein,2 (MK-3102), a Novel Once-Weekly Keith D. Kaufman,2 and Samuel S. Engel2 DPP-4 Inhibitor for the Treatment of Patients With Type 2 Diabetes Diabetes Care 2015;38:2106–2114 | DOI: 10.2337/dc15-0109 OBJECTIVE This study was conducted to determine the optimal dose of omarigliptin, a once- weekly (q.w.) dipeptidyl peptidase IV (DPP-4) inhibitor, for the treatment of patients with type 2 diabetes and evaluate the long-term safety of that dose. RESEARCH DESIGN AND METHODS In a multicenter, double-blind, 12-week, dose-range finding study, 685 oral antihy- perglycemic agent-na¨ıve or washed-out subjects with type 2 diabetes were random- ized to one of five once-weekly doses of omarigliptin (0.25 mg, 1 mg, 3 mg, 10 mg, or 25 mg) or placebo. The primary efficacy end point was change from baseline in HbA1c, and secondary end points were 2-h postmeal glucose (PMG) and fasting plasma glucose (FPG). Analysis included all patients who received at least one dose of the study medication. Subjects who completed the base study were eligible to enter a 66-week extension study. RESULTS Once-weekly treatment for 12 weeks with omarigliptin provided dose-related reduc- 1Division of Endocrinology and Metabolism, De- partment of Internal Medicine, Taichung Veterans EMERGING TECHNOLOGIES AND THERAPEUTICS tions in HbA , 2-h PMG, and FPG. -

Links Between HPA Axis and Adipokines: Clinical Implications in Paradigms of Stress-Related Disorders
Expert Review of Endocrinology & Metabolism ISSN: 1744-6651 (Print) 1744-8417 (Online) Journal homepage: https://www.tandfonline.com/loi/iere20 Links between HPA axis and adipokines: clinical implications in paradigms of stress-related disorders Panagiota Papargyri, Evangelia Zapanti, Nicolaos Salakos, Loukas Papargyris, Alexandra Bargiota & George MASTORAKOS To cite this article: Panagiota Papargyri, Evangelia Zapanti, Nicolaos Salakos, Loukas Papargyris, Alexandra Bargiota & George MASTORAKOS (2018) Links between HPA axis and adipokines: clinical implications in paradigms of stress-related disorders, Expert Review of Endocrinology & Metabolism, 13:6, 317-332, DOI: 10.1080/17446651.2018.1543585 To link to this article: https://doi.org/10.1080/17446651.2018.1543585 Accepted author version posted online: 01 Nov 2018. Published online: 13 Nov 2018. Submit your article to this journal Article views: 55 View related articles View Crossmark data Full Terms & Conditions of access and use can be found at https://www.tandfonline.com/action/journalInformation?journalCode=iere20 EXPERT REVIEW OF ENDOCRINOLOGY & METABOLISM 2018, VOL. 13, NO. 6, 317–332 https://doi.org/10.1080/17446651.2018.1543585 REVIEW Links between HPA axis and adipokines: clinical implications in paradigms of stress-related disorders Panagiota Papargyria, Evangelia Zapantib, Nicolaos Salakosc, Loukas Papargyrisd,e, Alexandra Bargiotaf and George MASTORAKOSa aUnit of Endocrinology, Diabetes Mellitus and Metabolism, Aretaieion Hospital, School of Medicine, National and Kapodistrian -
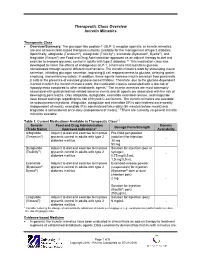
Therapeutic Class Overview Incretin Mimetics
Therapeutic Class Overview Incretin Mimetics Therapeutic Class Overview/Summary: The glucagon-like peptide-1 (GLP-1) receptor agonists, or incretin mimetics, are one of two incretin-based therapies currently available for the management of type 2 diabetes. Specifically, albiglutide (Tanzeum®), dulaglutide (Trulicity®), exenatide (Bydureon®, Byetta®), and liraglutide (Victoza®) are Food and Drug Administration-approved as an adjunct therapy to diet and exercise to improve glycemic control in adults with type 2 diabetes.1-5 This medication class was developed to mimic the effects of endogenous GLP-1, a hormone that maintains glucose homeostasis through several different mechanisms. The incretin mimetics work by stimulating insulin secretion, inhibiting glucagon secretion, improving β cell responsiveness to glucose, delaying gastric emptying, and enhancing satiety. In addition, these agents increase insulin secretion from pancreatic β cells in the presence of elevated glucose concentrations. Therefore, due to the glucose-dependent manner in which the incretin mimetics work, the medication class is associated with a low risk of hypoglycemia compared to other antidiabetic agents.6 The incretin mimetics are most commonly associated with gastrointestinal-related adverse events and all agents are associated with the risk of developing pancreatitis. Only albiglutide, dulaglutide, exenatide extended-release, and liraglutide have boxed warnings regarding the risk of thyroid C-cell tumors. The incretin mimetics are available as subcutaneous injections. Albiglutide, dulaglutide and exenatide ER is administered once-weekly (independent of meals), exenatide IR is administered twice-daily (60 minutes before meals) and liraglutide is administered once-daily (independent of meals).1-5 There are currently no generic incretin mimetics available. Table 1. -

Adiponectin Levels Associated with the Development of Hypertension: a Prospective Study
229 Hypertens Res Vol.31 (2008) No.2 p.229-233 Original Article Adiponectin Levels Associated with the Development of Hypertension: A Prospective Study Takuya IMATOH1), Motonobu MIYAZAKI2), Yoshito MOMOSE1), Shinichi TANIHARA1), and Hiroshi UNE1) Adiponectin is a recently discovered protein that seems to be exclusively secreted by adipocytes and is the most abundant adipose tissue–derived protein. While some recent studies have demonstrated an associa- tion between adiponectin levels and hypertension, these studies were cross-sectional in design, and the results have been inconsistent. Therefore we performed a prospective study to elucidate the role of adi- ponectin in the development of hypertension. The results of this study showed that serum adiponectin lev- els were significantly lower in hypertensive subjects than in normotensive subjects. Moreover, in logistic regression analysis, the subjects in the lowest quartile had a 3.72-fold higher risk than those in the highest quartile. Even after adjusting for potential confounding factors, this association was found to be significant. Low serum adiponectin levels were found to be independently associated with a higher risk for the devel- opment of hypertension. Our results therefore suggest that hypoadiponectinemia is a novel predictor of hypertension. (Hypertens Res 2008; 31: 229–233) Key Words: hypertension, adiponectin, prospective study, epidemiological study adipocytes and is the most abundant adipose tissue–derived 1 2 Introduction protein ( , ). Plasma adiponectin levels in humans are lower in obese than in non-obese subjects, in patients with coronary The latest World Health Organization (WHO) projections artery disease and diabetes mellitus type 2 than in healthy indicate that approximately 1.6 billion adults (aged ≥15 subjects, higher in women than in men. -

Delayed Response of Amylin Levels After an Oral Glucose Challenge in Children with Prader-Willi Syndrome
DOI 10.3349/ymj.2011.52.2.257 Original Article pISSN: 0513-5796, eISSN: 1976-2437 Yonsei Med J 52(2):257-262, 2011 Delayed Response of Amylin Levels after an Oral Glucose Challenge in Children with Prader-Willi Syndrome Hae Jeong Lee,1* Yon Ho Choe,2* Jee Hyun Lee,3 Young Bae Sohn,2 Su Jin Kim,2 Sung Won Park,2 Jun Seok Son,4 Seon Woo Kim,5 and Dong-Kyu Jin2 Departments of 1Pediatrics and 4Occupational and Environmental Medicine, Samsung Changwon Hospital, Sungkyunkwan University School of Medicine, Changwon; 2Department of Pediatrics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul; 3Department of Pediatrics, Kangnam Sacred Heart Hospital, Hallym University School of Medicine, Seoul; 5Clinical Research Center, Samsung Biomedical Research Institute, Seoul, Korea. Received: April 7, 2010 Purpose: Amylin secretion is increased parallel to insulin in obese subjects. Despite Revised: July 9, 2010 their marked obesity, a state of relative hypoinsulinemia occurs in children with Accepted: July 12, 2010 Prader-Willi syndrome (PWS). Based on the hypothesis that amylin levels may be Corresponding author: Dr. Dong-Kyu Jin, relatively low in PWS children, contributing to their excessive appetite, we studied Department of Pediatrics, Samsung Medical amylin levels after oral glucose loading in children with PWS and overweight con- Center, Sungkyunkwan University School of Medicine, 50 Irwon-dong, Gangnam-gu, trols. Materials and Methods: Plasma levels of amylin, glucagon, insulin, and glu- Seoul 135-710, Korea. cose were measured at 0, 30, 60, 90, and 120 min after a glucose challenge in chil- Tel: 82-2-3410-3525, Fax: 82-2-3410-0043 dren with PWS (n = 18) and overweight controls (n = 25); the relationships among E-mail: [email protected] the variables were investigated in these two groups. -

Therapeutic Class Overview Incretin Mimetics
Therapeutic Class Overview Incretin Mimetics Therapeutic Class • Overview/Summary: The glucagon-like peptide-1 (GLP-1) receptor agonists, or incretin mimetics, are one of two incretin-based therapies currently available for the management of type 2 diabetes. Specifically, albiglutide (Tanzeum®), exenatide (Bydureon®, Byetta®), and liraglutide (Victoza®) are Food and Drug Administration-approved as an adjunct therapy to diet and exercise to improve glycemic control in adults with type 2 diabetes.1-4 This medication class was developed to mimic the effects of endogenous GLP-1, a hormone that maintains glucose homeostasis through several different mechanisms. The incretin mimetics work by stimulating insulin secretion, inhibiting glucagon secretion, improving β cell responsiveness to glucose, delaying gastric emptying, and enhancing satiety. In addition, these agents increase insulin secretion from pancreatic β cells in the presence of elevated glucose concentrations. Therefore, due to the glucose-dependent manner in which the incretin mimetics work, the medication class is associated with a low risk of hypoglycemia compared to other antidiabetic agents.5,6 The incretin mimetics are most commonly associated with gastrointestinal-related adverse events, and all agents are associated with the risk of developing pancreatitis. Only albiglutide, exenatide extended-release, and liraglutide have boxed warnings regarding the risk of thyroid C-cell tumors. The incretin mimetics are available as subcutaneous injections. Albiglutide is administered -

Adiponectin and Resistin: Potential Metabolic Signals Affecting Hypothalamo-Pituitary Gonadal Axis in Females and Males of Different Species
REPRODUCTIONREVIEW PROOF ONLY Adiponectin and resistin: potential metabolic signals affecting hypothalamo-pituitary gonadal axis in females and males of different species Agnieszka Rak1, Namya Mellouk2, Pascal Froment2 and Joëlle Dupont2 1Department of Physiology and Toxicology of Reproduction, Institute of Zoology, Jagiellonian University in Krakow, Krakow, Poland and 2INRA, UMR 85 Physiologie de la Reproduction et des Comportements, Nouzilly, France Correspondence should be addressed to J Dupont; Email: [email protected] Abstract Adipokines, including adiponectin and resistin, are cytokines produced mainly by the adipose tissue. They play a significant role in metabolic functions that regulate the insulin sensitivity and inflammation. Alterations in adiponectin and resistin plasma levels, or their expression in metabolic and gonadal tissues, are observed in some metabolic pathologies, such as obesity. Several studies have shown that these two hormones and the receptors for adiponectin, AdipoR1 and AdipoR2 are present in various reproductive tissues in both sexes of different species. Thus, these adipokines could be metabolic signals that partially explain infertility related to obesity, such as polycystic ovary syndrome (PCOS). Species and gender differences in plasma levels, tissue or cell distribution and hormonal regulation have been reported for resistin and adiponectin. Furthermore, until now, it has been unclear whether adiponectin and resistin act directly or indirectly on the hypothalamo–pituitary–gonadal axis. The objective of this review was to summarise the latest findings and particularly the species and gender differences of adiponectin and resistin on female and male reproduction known to date, based on the hypothalamo–pituitary–gonadal axis. Reproduction (2017) 153 R215–R226 Introduction and pathological processes, such as food intake and metabolic control, diabetes, atherosclerosis, immunity Many authors have observed relationships between and also reproductive functions (Reverchon et al. -

New Insights Into Metabolic Homeostasis Keith Tan
Rockefeller University Digital Commons @ RU Student Theses and Dissertations 2015 Activity Based Profiling: New Insights into Metabolic Homeostasis Keith Tan Follow this and additional works at: http://digitalcommons.rockefeller.edu/ student_theses_and_dissertations Part of the Life Sciences Commons Recommended Citation Tan, Keith, "Activity Based Profiling: New Insights into Metabolic Homeostasis" (2015). Student Theses and Dissertations. Paper 285. This Thesis is brought to you for free and open access by Digital Commons @ RU. It has been accepted for inclusion in Student Theses and Dissertations by an authorized administrator of Digital Commons @ RU. For more information, please contact [email protected]. ACTIVITY BASED PROFILING: NEW INSIGHTS INTO METABOLIC HOMEOSTASIS A Thesis Presented to the Faculty of The Rockefeller University in Partial Fulfillment of the Requirements for the degree of Doctor of Philosophy by Keith Tan June 2015 © Copyright by Keith Tan 2015 ACTIVITY BASED PROFILING: NEW INSIGHTS INTO METABOLIC HOMEOSTASIS Keith Tan, Ph.D. The Rockefeller University 2015 There is mounting evidence that demonstrates that body weight and energy homeostasis is tightly regulated by a physiological system. This system consists of sensing and effector components that primarily reside in the central nervous system and disruption to these components can lead to obesity and metabolic disorders. Although many neural substrates have been identified in the past decades, there is reason to believe that there are numerous unidentified neural populations that play a role in energy balance. Besides regulating caloric consumption and energy expenditure, neural components that control energy homeostasis are also tightly intertwined with circadian rhythmicity but this aspect has received less attention.