Of the Tasman Sea, with Notes on the Tribe Heteromysini
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Policy Analysis of Invasive Species Regulations in Sydney Harbour and San Francisco Bay
California State University, Monterey Bay Digital Commons @ CSUMB Capstone Projects and Master's Theses 2005 Policy analysis of invasive species regulations in Sydney Harbour and San Francisco Bay Alexis Radovich California State University, Monterey Bay Christina Schmunk California State University, Monterey Bay Follow this and additional works at: https://digitalcommons.csumb.edu/caps_thes Recommended Citation Radovich, Alexis and Schmunk, Christina, "Policy analysis of invasive species regulations in Sydney Harbour and San Francisco Bay" (2005). Capstone Projects and Master's Theses. 54. https://digitalcommons.csumb.edu/caps_thes/54 This Capstone Project is brought to you for free and open access by Digital Commons @ CSUMB. It has been accepted for inclusion in Capstone Projects and Master's Theses by an authorized administrator of Digital Commons @ CSUMB. Unless otherwise indicated, this project was conducted as practicum not subject to IRB review but conducted in keeping with applicable regulatory guidance for training purposes. For more information, please contact [email protected]. Policy Analysis of Invasive Species Regulations in Sydney Harbour and San Francisco Bay A Capstone Project Presented to the Faculty of Science and Environmental Policy in the College of Science, Media Arts, and Technology at California State University, Monterey Bay in Partial Fulfilment of the Requirements for the Degree of Bachelor of Science by Alexis Radovich Christina Schmunk December 15, 2005 Policy Analysis of Invasive Species in Sydney Harbour, Australia and San Francisco Bay, United States of America. By Alexis Radovich and Christina Schmunk The demand for the mass transportation of goods throughout the world is threatening the biodiversity and native habitats of local scale aquatic assemblages. -

Two New Species of Heteromysis (Olivemysis) (Mysida, Mysidae
Zootaxa 2823: 32–46 (2011) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2011 · Magnolia Press ISSN 1175-5334 (online edition) Two new species of Heteromysis (Olivemysis) (Mysida, Mysidae, Heteromysinae) from the tropical northwest Atlantic with diagnostics on the subgenus Olivemysis Băcescu, 1968 W. WAYNE PRICE 1 & RICHARD W. HEARD2 1Department of Biology, University of Tampa, Tampa, Florida 33606, USA. E-mail: [email protected] 2Department of Coastal Sciences, University of Southern Mississippi, Ocean Springs, Mississippi 39566-7000, USA. E-mail: [email protected] Abstract A survey of mysid crustaceans in near-shore habitats of the Cayman Islands and the Turks and Caicos Islands, BWI yield- ed two new species of mysids belonging to the genus Heteromysis S. I. Smith, 1873. H. (Olivemysis) modlini n. sp. oc- curred on live bottom habitats in shallow waters of Grand Cayman Island, and H. (Olivemysis) mclellandi n. sp. from sponges in depths of 21–27 m on deep fringing reefs off Pine Cay, Turks and Caicos Islands. H. modlini may be distin- guished from closely related species in the western Atlantic by the following characters: (1) 6–7 robust flagellated setae on the medial margin of the carpo-propodus of thoracic endopod 3, (2) 3–5 and 4–6 bent, attenuated spines on male pleo- pods 3 and 4, respectively, (3) 3–4 spiniform setae along the medial margin of the uropodal endopod, and (4) 10–16 spinules along the anterior ¾ of the telsonic cleft, 14–19 spiniform setae completely lining the lateral margins of the tel- son, and each apical lobe of the telson with a pair of spiniform setae, the outer 1.6–2.0 times longer than the inner. -

Hawkesbury Shelf Environmental Background Report
HAWKESBURY SHELF MARINE BIOREGION ASSESSMENT Hawkesbury Shelf environmental background report Background The NSW Marine Estate Management Authority (the Authority) was established by the NSW Government in 2013 to advise on policies, priorities and directions for the NSW marine estate. The NSW marine estate includes marine waters, estuaries and the coast. It extends seaward out to three nautical miles and from the Queensland border in the north to the Victorian border in the south. The full definition and map can be found at www.marine.nsw.gov.au. Contributors The Authority acknowledges the key contributions of officers from the following in preparing this report: • NSW Department of Primary Industries • Office of Environment and Heritage • Transport for NSW • Department of Planning and Environment • Marine Estate Expert Knowledge Panel Published by the NSW Marine Estate Management Authority Hawkesbury Shelf marine bioregion assessment – Hawkesbury Environmental background report First published February 2016 ISBN 978-1-74256-893-5 More information This paper and more information about the Hawkesbury Shelf marine bioregion assessment are available at www.marine.nsw.gov.au. RM8 reference INT15/135530 © State of New South Wales through the Department of Industry, Skills and Regional Development, 2016.This publication is copyright. You may download, display, print and reproduce this material provided that the wording is reproduced exactly, the source is acknowledged, and the copyright, update address and disclaimer notice are retained. To copy, adapt, publish, distribute or commercialise any of this publication you will need to seek permission from the Department of Industry, Skills and Regional Development. Disclaimer: The information contained in this publication is based on knowledge and understanding at the time of writing (February 2016). -

From Catchment to Inner Shelf: Insights Into NSW Coastal Compartments
University of Wollongong Research Online Faculty of Science, Medicine and Health - Papers: part A Faculty of Science, Medicine and Health 1-1-2015 From catchment to inner shelf: insights into NSW coastal compartments Rafael Cabral Carvalho University of Wollongong, [email protected] Colin D. Woodroffe University of Wollongong, [email protected] Follow this and additional works at: https://ro.uow.edu.au/smhpapers Part of the Medicine and Health Sciences Commons, and the Social and Behavioral Sciences Commons Recommended Citation Cabral Carvalho, Rafael and Woodroffe, Colin D., "From catchment to inner shelf: insights into NSW coastal compartments" (2015). Faculty of Science, Medicine and Health - Papers: part A. 4632. https://ro.uow.edu.au/smhpapers/4632 Research Online is the open access institutional repository for the University of Wollongong. For further information contact the UOW Library: [email protected] From catchment to inner shelf: insights into NSW coastal compartments Abstract This paper addresses the coastal compartments of the eastern coast by analysing characteristics of the seven biggest catchments in NSW (Shoalhaven, Hawkesbury, Hunter, Manning, Macleay, Clarence and Richmond) and coastal landforms such as estuaries, sand barriers, beaches, headlands, nearshore and inner shelf, providing a framework for estimating sediment budgets by delineating compartment boundaries and defining management units. It sheds light on the sediment dispersal yb rivers and longshore drift by reviewing literature, using available information/data, and modelling waves and sediment dispersal. Compartments were delineated based on physical characteristics through interpretation of hydrologic, geomorphic, geophysical, sedimentological, oceanographic factors and remote sensing. Results include identification of 36 primary compartments along the NSW coast, 80 secondary compartments on the South and Central coast, and 5 tertiary compartments for the Shoalhaven sector. -
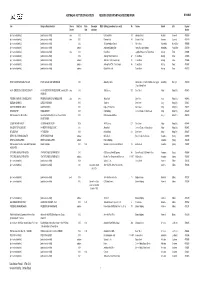
AIA REGISTER Jan 2015
AUSTRALIAN INSTITUTE OF ARCHITECTS REGISTER OF SIGNIFICANT ARCHITECTURE IN NSW BY SUBURB Firm Design or Project Architect Circa or Start Date Finish Date major DEM Building [demolished items noted] No Address Suburb LGA Register Decade Date alterations Number [architect not identified] [architect not identified] circa 1910 Caledonia Hotel 110 Aberdare Street Aberdare Cessnock 4702398 [architect not identified] [architect not identified] circa 1905 Denman Hotel 143 Cessnock Road Abermain Cessnock 4702399 [architect not identified] [architect not identified] 1906 St Johns Anglican Church 13 Stoke Street Adaminaby Snowy River 4700508 [architect not identified] [architect not identified] undated Adaminaby Bowling Club Snowy Mountains Highway Adaminaby Snowy River 4700509 [architect not identified] [architect not identified] circa 1920 Royal Hotel Camplbell Street corner Tumut Street Adelong Tumut 4701604 [architect not identified] [architect not identified] 1936 Adelong Hotel (Town Group) 67 Tumut Street Adelong Tumut 4701605 [architect not identified] [architect not identified] undated Adelonia Theatre (Town Group) 84 Tumut Street Adelong Tumut 4701606 [architect not identified] [architect not identified] undated Adelong Post Office (Town Group) 80 Tumut Street Adelong Tumut 4701607 [architect not identified] [architect not identified] undated Golden Reef Motel Tumut Street Adelong Tumut 4701725 PHILIP COX RICHARDSON & TAYLOR PHILIP COX and DON HARRINGTON 1972 Akuna Bay Marina Liberator General San Martin Drive, Ku-ring-gai Akuna Bay Warringah -

Marine Ecology
Appendix T Appendix T Marine ecology January 2020 Roads and Maritime Services Western Harbour Tunnel and Warringah Freeway Upgrade Technical working paper: Marine ecology January 2020 Prepared for Roads and Maritime Prepared by Cardno (NSW/ACT) Pty Ltd © Roads and Maritime The concepts and information contained in this document are the property of Roads and Maritime Services. You must not reproduce any part of this document without the prior written approval of Roads and Maritime Services. Table of Contents Executive Summary 1 1 Introduction 11 1.1 Overview 11 1.2 The project 11 1.3 Key construction activities 16 1.4 Project location 19 1.5 Purpose of this report 19 1.6 Secretary’s environmental assessment requirements 19 1.7 Avoid and minimise 19 1.8 Legislative context 20 1.9 Previous investigations for the project 21 1.10 Other project investigations 21 1.11 Definitions 22 2 Methods 24 2.1 Overview 24 2.2 Personnel 25 2.3 Background research 26 2.4 Field survey 26 2.5 Data analyses 31 2.6 Limitations 32 2.7 Impact assessment (including risk analysis) 33 3 Existing environment overview 36 3.1 Geology and socio-economics 36 3.2 Coastal processes and hydrology 36 3.3 Sediment properties 36 3.4 Water quality 38 3.5 Marine habitat types and communities 39 3.6 Threatened ecological communities 67 3.7 Critical habitat 67 3.8 Threatened marine species and endangered populations 67 3.9 Protected marine species 72 3.10 Matters of National Environmental Significance (MNES) 74 3.11 Wetlands and conservation areas 75 3.12 Key threatening processes -

Four New Species of Heteromysis (Crustacea: Mysida) from Public Aquaria in Hawaii, Florida, and Western to Central Europe
European Journal of Taxonomy 735: 133–175 ISSN 2118-9773 https://doi.org/10.5852/ejt.2021.735.1247 www.europeanjournaloftaxonomy.eu 2021 · Wittmann K.J. & Abed-Navandi D. This work is licensed under a Creative Commons Attribution License (CC BY 4.0). Research article urn:lsid:zoobank.org:pub:F1CE3697-319D-4D02-A99F-11A0E16A8743 Four new species of Heteromysis (Crustacea: Mysida) from public aquaria in Hawaii, Florida, and Western to Central Europe Karl J. WITTMANN 1,* & Daniel ABED-NAVANDI 2 1 Medical University of Vienna, Department of Environmental Health, Kinderspitalgasse 15, 1090 Vienna, Austria. 2 Haus des Meeres – Aqua Terra Zoo, Fritz Grünbaum Platz 1, 1060 Vienna, Austria. * Corresponding author: [email protected] 2 Email: [email protected] 1 urn:lsid:zoobank.org:author:C90E7BC4-A27A-4B41-93F3-6224D17795FF 2 urn:lsid:zoobank.org:author:179B83B0-8C8B-4DF5-8986-4227B8E1BB9B Abstract. Four new species of the subgenus Heteromysis (Olivemysis) were detected in material from (sub)-tropical aquaria in six public aquarium institutions around the globe. Modifications of pleopods by spines represent the strongest structural complex used for differentiation within this subgenus: male pleopods 1–4 modified in H. smithsoniana sp. nov., male pleopods 2–4 plus female pleopod 2 in H. hornimani sp. nov. and H. waikikensis sp. nov. Additional important diagnostic characters are provided by the antennulae, uropods, and telson. The male of H. sixi sp. nov. represents a very rare case within the genus Heteromysis by having only pleopod 2 modified by flagellate spines. The definition of the subgenus Olivemysis is modified in order to include H. -

R/Tme'icanjafuseum
r/tme'icanJAfuseum PUBLISHED BY THE AMERICAN MUSEUM OF NATURAL HISTORY CENTRAL PARK WEST AT 79TH STREET, NEW YORK 24, N.Y. NUMBER 1716 MARCH 25, 1955 A New Species of the Genus Heteromysis (Crustacea, Mysidacea) from the Bahama Islands, Commensal with a Sea-Anemone BY WM. D. CLARKE The material upon which this paper is based was collected at the Lerner Marine Laboratory, Bimini, Bahama Islands. The author wishes to ex- press his thanks to Dr. Digby McLaren for first pointing out this inter- esting mysid, and to Dr. Fenner Chace for his helpful advice during the preparation of this paper. Heteromysis actiniae, new species Figures 1, 2, 3, and 8 HOLOTYPE FEMALE: The general body form is robust and compact. The anterior margin of the carapace is produced into a broad, triangular rostrum, the tip of which is rounded and projects as far as the basal segment of the antennular peduncle. The cervical sulcus is apparent. The posterior margin of the carapace is deeply indented so as to expose dorsally the last two thoracic segments. The abdominal segments are of equal size, but the last is somewhat larger than its predecessors. The antennules and antennae do not demonstrate any appreciable dif- ferences from other species of the genus. The antennal scale is small (barely equals the length of the antennular peduncle) and setose around its entire margin. On the median surface of the distal segment of the antennular peduncle near the base of the inner flagellum there is a small inflated process terminated by a seta. This structure is present in both sexes of H. -

Annual & Sustainability Report
ANNUAL & SUSTAINABILITY REPORT 2009-10 Contents Letter from the President 4 Part 1: ACF’s Sustainability Scorecard 6 Part 2: Our Environmental and Social impact 6 Our sustainable practices 7 Our own wellbeing 7 Reaching out 9 Part 3: Our Campaigns 9 The Climate Project – Australia 9 The protection of Koongarra 9 Sustainable Cities Index 10 Just Add Water 10 Healthy oceans 10 Alwal National Park 11 Other campaigns: from red gums to green homes 12 Part 4: Our Supporters 17 Part 5: Financials 17 ACF board 23 Independent auditor’s report 25 Financials Dear ACF Supporter commit to keep warming to below two degrees. As we look back and refl ect on the year I was also impressed and proud when passed, the challenges met and the lessons 10 of our Climate Project presenters learned, I am reminded again of the joined with colleagues from the Union strength, resilience and commitment of Climate Connectors and the Australian ACF supporters. Youth Climate Coalition in Canberra. Never was this more evident to me They met with 56 MPs to tell them that than during the diffi cult Copenhagen millions of Australians care deeply about campaign, in which ACF supporters joined climate change and climate policies would together to lobby world leaders at the infl uence their votes in the federal election. UN Climate Conference. In the lead up, There are many ways supporters, like ACF campaigners worked hard to deliver you, contribute to ACF’s work protecting science-based policy to then Prime Minister Australia’s unique natural heritage. The Kevin Rudd and senior offi cials attending number of people regularly participating in the talks. -
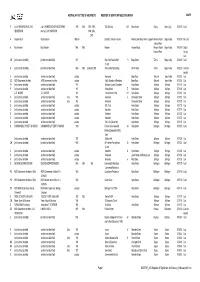
MASTER AIA Register of Significant Architecture February2021.Xls AUSTRALIAN INSTITUTE of ARCHITECTS REGISTER of SIGNIFICANT BUILDINGS in NSW MASTER
AUSTRALIAN INSTITUTE OF ARCHITECTS REGISTER OF SIGNIFICANT BUILDINGS IN NSW MASTER O A & K HENDERSON / LOUIS A & K HENDERSON OF MELBOURNE, 1935 1940 1991, 1993, T&G Building 555 Dean Street Albury Albury City 4703473Card HENDERSON rear by LOUIS HARRISON 1994, 2006, 2008 H Graeme Gunn Graeme Gunn 1968-69 Baronda (Yencken House) Nelson Lake Road, Nelson Lagoon Mimosa Rocks Bega Valley 4703519 No Card National Park H Roy Grounds Roy Grounds 1964 1980 Penders Haighes Road Mimosa Rocks Bega Valley 4703518 Digital National Park Listing Card CH [architect not identified] [architect not identified] 1937 Star of the Sea Catholic 19 Bega Street Tathra Bega Valley 4702325 Card Church G [architect not identified] [architect not identified] 1860 1862 Extended 2004 Tathra Wharf & Building Wharf Road Tathra Bega Valley 4702326 Card not located H [architect not identified] [architect not identified] undated Residence Bega Road Wolumla Bega Valley 4702327 Card SC NSW Government Architect NSW Government Architect undated Public School and Residence Bega Road Wolumla Bega Valley 4702328 Card TH [architect not identified] [architect not identified] 1911 Bellingen Council Chambers Hyde Street Bellingen Bellingen 4701129 Card P [architect not identified] [architect not identified] 1910 Federal Hotel 77 Hyde Street Bellingen Bellingen 4701131 Card I G. E. MOORE G. E. MOORE 1912 Former Masonic Hall 121 Hyde Street Bellingen Bellingen 4701268 Card H [architect not identified] [architect not identified] circa 1905 Residence 4 Coronation Street Bellingen Bellingen -

Invertebrate ID Guide
11/13/13 1 This book is a compilation of identification resources for invertebrates found in stomach samples. By no means is it a complete list of all possible prey types. It is simply what has been found in past ChesMMAP and NEAMAP diet studies. A copy of this document is stored in both the ChesMMAP and NEAMAP lab network drives in a folder called ID Guides, along with other useful identification keys, articles, documents, and photos. If you want to see a larger version of any of the images in this document you can simply open the file and zoom in on the picture, or you can open the original file for the photo by navigating to the appropriate subfolder within the Fisheries Gut Lab folder. Other useful links for identification: Isopods http://www.19thcenturyscience.org/HMSC/HMSC-Reports/Zool-33/htm/doc.html http://www.19thcenturyscience.org/HMSC/HMSC-Reports/Zool-48/htm/doc.html Polychaetes http://web.vims.edu/bio/benthic/polychaete.html http://www.19thcenturyscience.org/HMSC/HMSC-Reports/Zool-34/htm/doc.html Cephalopods http://www.19thcenturyscience.org/HMSC/HMSC-Reports/Zool-44/htm/doc.html Amphipods http://www.19thcenturyscience.org/HMSC/HMSC-Reports/Zool-67/htm/doc.html Molluscs http://www.oceanica.cofc.edu/shellguide/ http://www.jaxshells.org/slife4.htm Bivalves http://www.jaxshells.org/atlanticb.htm Gastropods http://www.jaxshells.org/atlantic.htm Crustaceans http://www.jaxshells.org/slifex26.htm Echinoderms http://www.jaxshells.org/eich26.htm 2 PROTOZOA (FORAMINIFERA) ................................................................................................................................ 4 PORIFERA (SPONGES) ............................................................................................................................................... 4 CNIDARIA (JELLYFISHES, HYDROIDS, SEA ANEMONES) ............................................................................... 4 CTENOPHORA (COMB JELLIES)............................................................................................................................ -
Teleostei, Gobiesocidae)
A peer-reviewed open-access journal ZooKeys A864: new 35–65 genus (2019) and two new species of miniature clingfishes from temperate southern Australia 35 doi: 10.3897/zookeys.864.34521 RESEARCH ARTICLE http://zookeys.pensoft.net Launched to accelerate biodiversity research A new genus and two new species of miniature clingfishes from temperate southern Australia (Teleostei, Gobiesocidae) Kevin W. Conway1,2, Glenn I. Moore3,4, Adam P. Summers5,6 1 Department of Wildlife and Fisheries Sciences and Biodiversity Research and Teaching Collections, Texas A&M University, College Station, TX 77843, USA 2 Research Associate, Ichthyology, Australian Museum Research Institute, 1 William Street, Sydney, NSW 2010, Australia 3 Fish Section, Department of Aquatic Zoology, Western Australian Museum, Locked Bag 49 Welshpool DC WA 6986, Australia 4 School of Biolo- gical Sciences, University of Western Australia, Nedlands, WA 6907, Australia 5 Friday Harbor Laboratories, University of Washington, Friday Harbor, Washington 98250, USA 6 Burke Museum of Natural History and Culture, University of Washington, Seattle, WA 98105, USA Corresponding author: Kevin W. Conway ([email protected]) Academic editor: David Morgan | Received 15 March 2019 | Accepted 31 May 2019 | Published 15 July 2019 http://zoobank.org/5B236AA0-725A-478D-96D4-6B8F366126D4 Citation: Conway KW, Moore GI, Summers AP (2019) A new genus and two new species of miniature clingfishes from temperate southern Australia (Teleostei, Gobiesocidae). ZooKeys 864: 35–65. https://doi.org/10.3897/ zookeys.864.34521 Abstract A new genus and two new species of miniature clingfishes are described based on specimens collected from dense stands of macroalgae in intertidal and shallow subtidal areas along the coast of southern Australia.