Interpretation of P16 Immunohistochemistry in Lower Anogenital Tract Neoplasia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Genetic Markers in Lung Cancer Diagnosis: a Review
International Journal of Molecular Sciences Review Genetic Markers in Lung Cancer Diagnosis: A Review Katarzyna Wadowska 1 , Iwona Bil-Lula 1 , Łukasz Trembecki 2,3 and Mariola Sliwi´ ´nska-Mosso´n 1,* 1 Department of Medical Laboratory Diagnostics, Division of Clinical Chemistry and Laboratory Haematology, Wroclaw Medical University, 50-556 Wroclaw, Poland; [email protected] (K.W.); [email protected] (I.B.-L.) 2 Department of Radiation Oncology, Lower Silesian Oncology Center, 53-413 Wroclaw, Poland; [email protected] 3 Department of Oncology, Faculty of Medicine, Wroclaw Medical University, 53-413 Wroclaw, Poland * Correspondence: [email protected]; Tel.: +48-71-784-06-30 Received: 1 June 2020; Accepted: 25 June 2020; Published: 27 June 2020 Abstract: Lung cancer is the most often diagnosed cancer in the world and the most frequent cause of cancer death. The prognosis for lung cancer is relatively poor and 75% of patients are diagnosed at its advanced stage. The currently used diagnostic tools are not sensitive enough and do not enable diagnosis at the early stage of the disease. Therefore, searching for new methods of early and accurate diagnosis of lung cancer is crucial for its effective treatment. Lung cancer is the result of multistage carcinogenesis with gradually increasing genetic and epigenetic changes. Screening for the characteristic genetic markers could enable the diagnosis of lung cancer at its early stage. The aim of this review was the summarization of both the preclinical and clinical approaches in the genetic diagnostics of lung cancer. The advancement of molecular strategies and analytic platforms makes it possible to analyze the genome changes leading to cancer development—i.e., the potential biomarkers of lung cancer. -
In Women with the Two Groups,3 When Judged by Colposcopy in a Relative
Matters arising 145 4 Reynolds JEF (Ed) Martindale. The Extra Phar- There are a number of small points in With regard to the points raised by Drs macoepia, 29th ed. London: Pharmaceutical respect ofthe data they present which require Evans and Kell. Patients attending our clinic Press. 1989. 5 National Institute of Occupational Safety and clarification: the indications for taking a are offered cervical cytology if (a) they have Hazards. Recommendations for occupational cervical smear are actually not given and it is not had a smear within the last 3 years or (b) safety and health standards. 1988. Morbidity not clear whether the 185 patients represent they or their sexual partners have genital and mortality weekly report (suppl) warts and they have not had a smear within Genitourin Med: first published as 10.1136/sti.68.2.145-b on 1 April 1992. Downloaded from 1988;37:8. the total number smeared over the 5 month 6 Ethyl chloride BP. Production Information. period of study. It is really quite important to one year. The 185 women in the study were Berkshire UK. Bengue and Company Ltd know who was invited to participate and who drawn from 191 women having smears dur- 1982. declined. ing the study period. No patients declined to 7 Nordin C, Rosenguist M, Hollstedt C. Sniffing of ethyl chloride-an uncommon form of The proportion of abnormal smears was answer the life-style questions, but six abuse with serious mental and neurological much lower in the non-wart group (7 of 55) patients, all from the warts/warts contact symptoms. -

Invasive Cervical Cancer Audit; EU Guidelines for Quality Assurance
The 4th EFCS Annual Tutorial Ospedale Universitario di Cattinara, Strada di Fiume, Trieste Handouts for lectures and workshops – I I - Gynaecological cytopathology Mrs Rietje Salet‐van‐de Pol, Radboud University Nijmegen Medical Centre, Nijmegen, The Netherlands Gynecological cytology: technical aspects ............................................................................... 2 Non‐neoplastic gynecological cytology .................................................................................... 6 • Dr Giovanni Negri, General Hospital of Bolzano, Bozano SIL and cancer; ASC‐US, ASC‐H, diagnostic pitfalls and look‐alikes; glandular abnormalities 11 • Dr Amanda Herbert, Guy’s & St Thomas’ NHS Foundation Trust, London Invasive cervical cancer audit; EU guidelines for quality assurance ...................................... 17 1 Gynecological cytology: technical aspects Rietje Salet-van de Pol Important in specimen processing is to obtain as much as possible well preserved cells for microscopically evaluation. The quality of the smear depends on cell sampling, fixation and staining. For obtaining enough cervical material you are dependent on the cell sampler. For cervical cytology two types of specimen are available: conventional smears and liquid based cytology (LBC). Conventional, Thinprep and Surepath slides In conventional cytology the cell sampler makes the smear and is responsible for the fixation of the cells. Reasons for unsatisfactory conventional smears can be obscuring blood or inflammatory cells, thick smears with overlapping cells, poor preservation of the cells due to late fixation and low cellularity. In LBC the cell sampler immediately transferred the cellular material into a vial with fixative (fixating solution) which gives a better preservation of the cells. The laboratory is responsible for processing of the smear. LBC gives equally distribution of the cells in a thin cell layer of well preserved cells. The rate of unsatisfactory smears is lower. -

The P16 (Cdkn2a/Ink4a) Tumor-Suppressor Gene in Head
The p16 (CDKN2a/INK4a) Tumor-Suppressor Gene in Head and Neck Squamous Cell Carcinoma: A Promoter Methylation and Protein Expression Study in 100 Cases Lingbao Ai, M.D., Krystal K. Stephenson, Wenhua Ling, M.D., Chunlai Zuo, M.D., Perkins Mukunyadzi, M.D., James Y. Suen, M.D., Ehab Hanna, M.D., Chun-Yang Fan, M.D., Ph.D. Departments of Pathology (LA, KKS, CZ, PM, CYF) and Otolaryngology-Head and Neck Surgery (CYF, JYS, EH), University of Arkansas for Medical Sciences; and School of Public Health (LA, WL), Sun-Yat Sen University, Guangzhou, China apparent loss of p16 protein expression appears to The p16 (CDKN2a/INK4a) gene is an important be an independent prognostic factor, although loss tumor-suppressor gene, involved in the p16/cyclin- of p16 protein may be used to predict overall pa- dependent kinase/retinoblastoma gene pathway of tient survival in early-stage head and neck squa- cell cycle control. The p16 protein is considered to mous cell carcinoma. be a negative regulator of the pathway. The gene encodes an inhibitor of cyclin-dependent kinases 4 KEY WORDS: Gene inactivation, Head and and 6, which regulate the phosphorylation of reti- neck squamous cell carcinoma, p16, Promoter noblastoma gene and the G1 to S phase transition of hypermethylation. the cell cycle. In the present study, p16 gene pro- Mod Pathol 2003;16(9):944–950 moter hypermethylation patterns and p16 protein expression were analyzed in 100 consecutive un- The development of head and neck squamous cell treated cases of primary head and neck squamous carcinoma is believed to be a multistep process, in cell carcinoma by methylation-specific PCR and im- which genetic and epigenetic events accumulate as munohistochemical staining. -
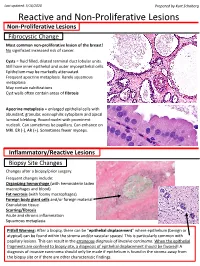
Reactive and Non-Proliferative Lesions
Last updated: 5/16/2020 Prepared by Kurt Schaberg Reactive and Non-Proliferative Lesions Non-Proliferative Lesions Fibrocystic Change Most common non-proliferative lesion of the breast! No significant increased risk of cancer. Cysts = fluid filled, dilated terminal duct lobular units. Still have inner epithelial and outer myoepithelial cells. Epithelium may be markedly attenuated. Frequent apocrine metaplasia. Rarely squamous metaplasia May contain calcifications Cyst walls often contain areas of fibrosis Apocrine metaplasia = enlarged epithelial cells with abundant, granular, eosinophilic cytoplasm and apical luminal blebbing. Round nuclei with prominent nucleoli. Can sometimes be papillary. Can enhance on MRI. ER (-), AR (+). Sometimes fewer myoeps. Inflammatory/Reactive Lesions Biopsy Site Changes Changes after a biopsy/prior surgery. Frequent changes include: Organizing hemorrhage (with hemosiderin laden macrophages and blood) Fat necrosis (with foamy macrophages) Foreign body giant cells and/or foreign material Granulation tissue Scarring/fibrosis Acute and chronic inflammation Squamous metaplasia Pitfall Warning: After a biopsy, there can be “epithelial displacement” where epithelium (benign or atypical) can be found within the stroma and/or vascular spaces! This is particularly common with papillary lesions. This can result in the erroneous diagnosis of invasive carcinoma. When the epithelial fragments are confined to biopsy site, a diagnosis of epithelial displacement should be favored! A diagnosis of invasive carcinoma should -

Primary Immature Teratoma of the Thigh Fig
CORRESPONDENCE 755 8. Gray W, Kocjan G. Diagnostic Cytopathology. 2nd ed. London: Delete all that do not apply: Elsevier Health Sciences, 2003; 677. 9. Richards A, Dalrymple C. Abnormal cervicovaginal cytology, unsatis- Cervix, colposcopic biopsy/LLETZ/cone biopsy: factory colposcopy and the use of vaginal estrogen cream: an obser- vational study of clinical outcomes for women in low estrogen states. Diagnosis: NIL (No intraepithelial lesion WHO 2014) J Obstet Gynaecol Res 2015; 41: 440e4. LSIL (CIN 1 with HPV effect WHO 2014) 10. Darragh TM, Colgan TJ, Cox T, et al. The lower anogenital squamous HSIL (CIN2/3 WHO 2014) terminology standardization project for HPV-associated lesions: back- Squamous cell carcinoma ground and consensus recommendation from the College of American Immature squamous metaplasia Pathologists and the American Society for Colposcopy and Cervical Adenocarcinoma in situ (AIS, HGGA) e Adenocarcinoma Pathology. Arch Pathol Lab Med 2012; 136: 1267 97. Atrophic change 11. McCluggage WG. Endocervical glandular lesions: controversial aspects e Extending into crypts: Not / Idenfied and ancillary techniques. J Clin Pathol 2013; 56: 164 73. Epithelial stripping: Not / Present 12. World Health Organization (WHO). Comprehensive Cervical Cancer Invasive disease: Not / Idenfied / Micro-invasive Control: A Guide to Essential Practice. 2nd ed. Geneva: WHO, 2014. Depth of invasion: mm Transformaon zone: Not / Represented Margins: DOI: https://doi.org/10.1016/j.pathol.2019.07.014 Ectocervical: Not / Clear Endocervical: Not / Clear Circumferenal: Not / Clear p16 status: Negave / Posive Primary immature teratoma of the thigh Fig. 3 A proposed synoptic reporting format for pathologists reporting colposcopic biopsies and cone biopsies or LLETZ. Sir, Teratomas are germ cell tumours composed of a variety of HSIL, AIS, micro-invasive or more advanced invasive dis- somatic tissues derived from more than one germ layer 12 ease. -

Involvement of the Cyclin-Dependent Kinase Inhibitor P16 (Ink4a) in Replicative Senescence of Normal Human Fibroblasts
Proc. Natl. Acad. Sci. USA Vol. 93, pp. 13742–13747, November 1996 Biochemistry Involvement of the cyclin-dependent kinase inhibitor p16 (INK4a) in replicative senescence of normal human fibroblasts DAVID A. ALCORTA*†,YUE XIONG‡,DAWN PHELPS‡,GREG HANNON§,DAVID BEACH§, AND J. CARL BARRETT* *Laboratory of Molecular Carcinogenesis, National Institute of Environmental Health Sciences, Research Triangle Park, NC 27709; ‡Lineberger Comprehensive Cancer Center, University of North Carolina, Chapel Hill, NC 27599; and §Howard Hughes Medical Institute, Cold Spring Harbor Laboratories, Cold Spring Harbor, NY 11724 Communicated by Raymond L. Erickson, Harvard University, Cambridge, MA, September 19, 1996 (received for review on May 15, 1996) ABSTRACT Human diploid fibroblasts (HDFs) can be viewed in ref. 5). In senescent fibroblasts, CDK2 is catalytically grown in culture for a finite number of population doublings inactive and the protein down-regulated (7). CDK4 is also before they cease proliferation and enter a growth-arrest state reported to be down-regulated in senescent cells (8), while the termed replicative senescence. The retinoblastoma gene prod- status of CDK6 has not been previously addressed. The uct, Rb, expressed in these cells is hypophosphorylated. To activating cyclins for these CDKs, cyclins D1 and E, are present determine a possible mechanism by which senescent human in senescent cells at similar or elevated levels relative to early fibroblasts maintain a hypophosphorylated Rb, we examined passage cells (8). A role of the CDK inhibitors in senescence the expression levels and interaction of the Rb kinases, CDK4 was revealed by the isolation of a cDNA of a highly expressed and CDK6, and the cyclin-dependent kinase inhibitors p21 message in senescent cells that encoded the CDK inhibitor, p21 and p16 in senescent HDFs. -

Transcriptional Regulation of the P16 Tumor Suppressor Gene
ANTICANCER RESEARCH 35: 4397-4402 (2015) Review Transcriptional Regulation of the p16 Tumor Suppressor Gene YOJIRO KOTAKE, MADOKA NAEMURA, CHIHIRO MURASAKI, YASUTOSHI INOUE and HARUNA OKAMOTO Department of Biological and Environmental Chemistry, Faculty of Humanity-Oriented Science and Engineering, Kinki University, Fukuoka, Japan Abstract. The p16 tumor suppressor gene encodes a specifically bind to and inhibit the activity of cyclin-CDK specific inhibitor of cyclin-dependent kinase (CDK) 4 and 6 complexes, thus preventing G1-to-S progression (4, 5). and is found altered in a wide range of human cancers. p16 Among these CKIs, p16 plays a pivotal role in the regulation plays a pivotal role in tumor suppressor networks through of cellular senescence through inhibition of CDK4/6 activity inducing cellular senescence that acts as a barrier to (6, 7). Cellular senescence acts as a barrier to oncogenic cellular transformation by oncogenic signals. p16 protein is transformation induced by oncogenic signals, such as relatively stable and its expression is primary regulated by activating RAS mutations, and is achieved by accumulation transcriptional control. Polycomb group (PcG) proteins of p16 (Figure 1) (8-10). The loss of p16 function is, associate with the p16 locus in a long non-coding RNA, therefore, thought to lead to carcinogenesis. Indeed, many ANRIL-dependent manner, leading to repression of p16 studies have shown that the p16 gene is frequently mutated transcription. YB1, a transcription factor, also represses the or silenced in various human cancers (11-14). p16 transcription through direct association with its Although many studies have led to a deeper understanding promoter region. -

Top Tips in 2 Minutes: Human Papilloma Virus (HPV) Vaccines
Mike Fitzpatrick Carry on screening The vog ue for screening tests, driven by discuss any findings with your GP’. powerful commercial and political forces, The popular appeal of screening tests in an Top Tips in is having an increasingly malign influence anxious age results from the inflation to on our patients’ health (as well as mythical status of the commonsensical imposing a growing burden on our notion that early detection leads to a more 2 minutes surgeries). favourable outcome. But this is only true if In recent weeks, two patients have early treatment is effective: this has not The Department of Health has chosen the presented me with the results of some of been demonstrated, for example, in bivalent vaccine Cervarix ™ for its national the latest screening initiatives in the private relation to prostate cancer or in the case of vaccination programme in England. Although sector. One had paid around £3000 for the atheromatous carotid arteries. There is a this will protect against human papilloma virus ‘ultimate check-up’. 1 In addition to related presumption that late presentation (HPV) 16 and 18, which cause 70% of cervical consultation and examination, the check- is a common factor resulting in a rapid cancers, it will offer no protection against up included ‘over 40’ blood and urine demise, particularly from cancer, but again, tests, audiometry, ECG and spirometry, this has to be substantiated, especially genital warts. In 2006, there were 83 745 new and ultrasound examinations of all internal when it may be the case that delays and diagnoses of genital warts (first episode) and organs. -

The P16 Status of Tumor Cell Lines Identifies Small Molecule Inhibitors Specific for Cyclin-Dependent Kinase 41
Vol. 5, 4279–4286, December 1999 Clinical Cancer Research 4279 The p16 Status of Tumor Cell Lines Identifies Small Molecule Inhibitors Specific for Cyclin-dependent Kinase 41 Akihito Kubo,2 Kazuhiko Nakagawa,2, 3 CDK4 kinase inhibitors that may selectively induce growth Ravi K. Varma, Nicholas K. Conrad, inhibition of p16-altered tumors. Jin Quan Cheng, Wen-Ching Lee, INTRODUCTION Joseph R. Testa, Bruce E. Johnson, INK4A 4 The p16 gene (also known as CDKN2A) encodes p16 , Frederic J. Kaye, and Michael J. Kelley which inhibits the CDK45:cyclin D and CDK6:cyclin D com- Medicine Branch [A. K., K. N., N. K. C., F. J. K., B. E. J.] and plexes (1). These complexes mediate phosphorylation of the Rb Developmental Therapeutics Program [R. K. V.], National Cancer Institute, Bethesda, Maryland 20889; Department of Medical protein and allow cell cycle progression beyond the G1-S-phase Oncology, Fox Chase Cancer Center, Philadelphia, Pennsylvania checkpoint (2). Alterations of p16 have been described in a wide 19111 [J. Q. C., W-C. L., J. R. T.]; and Department of Medicine, variety of histological types of human cancers including astro- Duke University Medical Center, Durham, North Carolina 27710 cytoma, melanoma, leukemia, breast cancer, head and neck [M. J. K.] squamous cell carcinoma, malignant mesothelioma, and lung cancer. Alterations of p16 can occur through homozygous de- ABSTRACT letion, point mutation, and transcriptional suppression associ- ated with hypermethylation in cancer cell lines and primary Loss of p16 functional activity leading to disruption of tumors (reviewed in Refs. 3–5). the p16/cyclin-dependent kinase (CDK) 4:cyclin D/retino- Whereas the Rb gene is inactivated in a narrow range of blastoma pathway is the most common event in human tumor cells, the pattern of mutational inactivation of Rb is tumorigenesis, suggesting that compounds with CDK4 ki- inversely correlated with p16 alterations (6–8), suggesting that nase inhibitory activity may be useful to regulate cancer cell a single defect in the p16/CDK4:cyclin D/Rb pathway is suffi- growth. -

(HPV) Induced Cervical Dysplasia
Cent. Eur. J. Biol.• 5(5) • 2010 • 554-571 DOI: 10.2478/s11535-010-0051-z Central European Journal of Biology Diagnostic role of p16/INK4A protein in Human Papillomavirus (HPV) induced cervical dysplasia Review Article Július Rajčáni1,2,*, Marián Adamkov1,3, Jana Hybenova1, Jaroslav Jackuliak1, Marian Benčat1 1Alpha Medical a.s., 03601 Martin, Slovak Republic 2Institute of Virology, Slovak Academy of Sciences, 84505 Bratislava, Slovak Republic 3Institute of Histology and Embryology, Jessenius Faculty of Medicine, Comenius University, 03601 Martin, Slovak Republic Received 15 October 2009; Accepted 13 April 2010 Abstract: The p16/INK4A protein is a cellular regulatory polypeptide over-expressed in the presence of high levels of the Human Papillomavirus (HPV) coded E7 protein. This review outlines the use of p16 antigen staining in cervical biopsies as well as in PAP smears summarizing the corresponding literature and commenting the authors’ own experience. The p16 antigen is a reliable marker for dysplastic cells in CINII/CINIII (HSIL) lesions as viewed in cervical biopsies. When PAP smears were examined at large scale screening for p16 antigen- reactive and atypical cells, considerable variations could be found especially in ASCUS graded lesions. Therefore, the presence of p16-reactive atypical cells in PAP smears should be interpreted together with the cytological signs of dysplasia, such as the altered N/C ratio. In addition, women revealing p16-positive ASCUS/LSIL specimens should be examined for the presence of HPV DNA. Detection of HPV DNA alone, i.e. in the absence of cytological screening has a low predictive value, since the clearance of HPV may occur even in the absence of morphological alterations. -

Squamous Metaplasia of the Tracheal Epithelium in Children
Thorax: first published as 10.1136/thx.31.2.167 on 1 April 1976. Downloaded from Thorax (1976), 31, 167. Squamous metaplasia of the tracheal epithelium in children AVINASH MITHAL' and JOHN L. EMERY2 The Chest Clinic, Lincoln' and The Children's Hospital, Sheffield' Mithal, A. and Emery, J. L. (1976). Thorax, 31, 167-171. Squamous metaplasia of the tracheal epithelium in children. Thirty-seven (16%) tracheas from 2170 children showed squamous metaplasia. (Cases with tracheo-oesophageal fistula and congenital heart disease were excluded.) The metaplasia extended into the bronchi in 15 cases. Features of pulmonary retention were present in seven cases. Respiratory infection, probably viral, seemed to be the most significant causative factor in 20 children, including those with cystic fibrosis. Tracheal instrumentation was a possible factor in 11 cases but oxygen therapy alone did not seem important. The metaplasia was almost certainly congenital in one child and probably in two others but no stillborn infants showed metaplasia. In many children the metaplasia seemed to be due to a combination of factors. Squamous metaplasia of the trachea in childhood Tracheas from children with tracheo-oesophageal has been described in cases of measles (Gold- fistula and those with congenital heart disease or zieher, 1918), influenza (Askanazy, 1919), cystic other gross deformities were excluded. There were fibrosis of the pancreas (Zuelzer and Newton, thus 2331 tracheas available for study. Epithelium 1949), and following intubation of the trachea was absent in 16 cases. This left 2170 tracheas for http://thorax.bmj.com/ (Rasche and Kuhns, 1972) and tracheostomy histological analysis. (Sara, 1967; Sara and Reye, 1969).