114- Jchps 10(1)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Khadi Institution Profile Khadi and Village Industries Comission
KHADI AND VILLAGE INDUSTRIES COMISSION KHADI INSTITUTION PROFILE Office Name : DO MADURAI TAMIL NADU Institution Code : 3274 Institution Name : RAMANATHAPURAM CENTRAL SARVODAYA SANGH Address: : 6/774, Arumugam Colony., SACHIYAPURAM Post : SIVAKASI West. City/Village : VIRUDHUNAGAR Pincode : 626124 State : TAMIL NADU District : VIRUDHUNAGAR Aided by : KVIC District : A+ Contact Person Name Email ID Mobile No. Chairman : K.KALIAPPAN [email protected] 9489056900 Secretary : P.RAVICHANDRAN [email protected] 9489056903 Nodal Officer : Registration Detail Registration Date Registration No. Registration Type 22-06-1977 18/1977 SOC Khadi Certificate No. TND/3211 Date : 31-MAR_2021 Khadi Mark No. KVIC/ZKMC/TN/017 Khadi Mark Dt. 01-Oct-2024 Sales Outlet Details Type Name Address City Pincode Sales Outlet GRAMA SHILPHA 7/21,SANKARANKOV SATTUR 626201 IL MAIN ROAD Production cum Sales KHADI VASTRALAYA 1/36,MUTHALIYAR VEMBAKOTTAI 626140 Outlet STREET Sales Outlet GRAMSHILPHA 1A/4 NADAR VIRUDHUNAGAR 626130 COMPLEX,THIRUTH ANGAL, Sales Outlet KHADI BHAVAN 6/818 -A SIVAKASI 626124 ARUMUGAM COLONY Sales Outlet GRAMA SHILPHA 3/672,TNC MUKKU VIRUDHUNAGAR 626144 ROAD Sales Outlet GRAMA SHILPHA 2/154,VIRUDHUNAG SIVAKASI 626103 AR MAIN ROAD CENTRAL 6/774,ARUMUGAM SIVAKASI 626124 VASTRALAYA,KHADI COLONY Sales Outlet KHADI GRAMODYOG 201, NEW ROAD, SIVAKASI 626123 BHAVAN Sales Outlet FURNITURE SHOWROOM 2/160A,ENCHAR SIVAKASI 626124 CROSS ROAD Production cum Sales KHADI VASTRALAYA 4/870,MAIN ROAD SANKARANKOVIL 627753 Outlet SILK PRODUCTION CENTER 1,MF, JAINAGAR -

P.S.R.Rengasamy College of Engineering for Women, Sivakasi-626
CONFERENCE TOPICS SUBMISSION GUIDELINE P.S.R.Rengasamy College of Engineering CSE & IT Paper Format : IEEE A4 limited to 5 pages for Women, Sivakasi-626 140 An ISO 9001: 2008 CERTIFIED INSTITUTION Soft Computing With single line spacing Approved by AICTE, New Delhi, Affiliated to Anna University Appayanaickenpatti, Cloud Computing Font Type : Times New Roman Sivakasi – 626 140. Grid Computing 6th National Conference Paper Title : Font Size 14 Points on P2P Computing Author Name(s) : Font Size 12 Points Recent Trends in Electrical, Electronics, Mobile Ad hoc Networks Communication & Computing Technologies Sub Title : 11 Points [NCRTE2C2T-2K17] Sensor Networks Text : 10 Points with Sub Title Bold ECE & EEE 24-03-2017 Embedded Systems ORGANIZING COMMITTEE REGISTRATION FORM Digital Signal Processing Chief Patron : Correspondent - Thiru. R.Solaisamy VLSI Technology Name : Co-Patron : Director - Er.S.Vigneshwari Image Processing Qualification : Convener : Principal - Dr.K.Ramasamy Designation : Sex : Power Systems Modeling Co-ordinators : Mr.P.Suresh Pandiarajan,HOD/ECE Experience : Teaching ……… Yrs, Industry …… Yrs Wireless Communication Mr.G.Sivakumar AP/ECE Organization : Satellite Communication Mr.K.karthik AP/ECE Communication Address Renewable Energy Sources Mr.N.R.Sathiskumar AP/ECE Pin: HVDC / FACTS Phone numbers (With STD Code) Power Electronics and Drives MAILING ADDRESS (Off) Special Electrical Machines The Co-ordinator(s) (Res) Call for Papers NCRTE2C2T-2K17 Mobile : Authors are requested to submit full P.S.R.Rengasamy College of Engineering for Women E-mail : paper with abstract through e-mail on or before Appayanaickenpatti, Sivakasi -626140 Details of Registration Fee: 16/03/2017 Phone No: 04562 – 239086 Mobile: 9884115995, Draft No…………....Dt…………….Amt.Rs………… 7708304530, 9894284422, 9750193401 Name of the Bank…………………………................. -
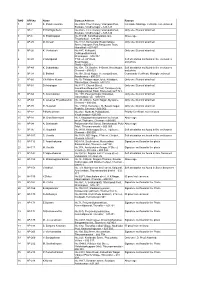
SNO APP.No Name Contact Address Reason 1 AP-1 K
SNO APP.No Name Contact Address Reason 1 AP-1 K. Pandeeswaran No.2/545, Then Colony, Vilampatti Post, Intercaste Marriage certificate not enclosed Sivakasi, Virudhunagar – 626 124 2 AP-2 P. Karthigai Selvi No.2/545, Then Colony, Vilampatti Post, Only one ID proof attached. Sivakasi, Virudhunagar – 626 124 3 AP-8 N. Esakkiappan No.37/45E, Nandhagopalapuram, Above age Thoothukudi – 628 002. 4 AP-25 M. Dinesh No.4/133, Kothamalai Road,Vadaku Only one ID proof attached. Street,Vadugam Post,Rasipuram Taluk, Namakkal – 637 407. 5 AP-26 K. Venkatesh No.4/47, Kettupatti, Only one ID proof attached. Dokkupodhanahalli, Dharmapuri – 636 807. 6 AP-28 P. Manipandi 1stStreet, 24thWard, Self attestation not found in the enclosures Sivaji Nagar, and photo Theni – 625 531. 7 AP-49 K. Sobanbabu No.10/4, T.K.Garden, 3rdStreet, Korukkupet, Self attestation not found in the enclosures Chennai – 600 021. and photo 8 AP-58 S. Barkavi No.168, Sivaji Nagar, Veerampattinam, Community Certificate Wrongly enclosed Pondicherry – 605 007. 9 AP-60 V.A.Kishor Kumar No.19, Thilagar nagar, Ist st, Kaladipet, Only one ID proof attached. Thiruvottiyur, Chennai -600 019 10 AP-61 D.Anbalagan No.8/171, Church Street, Only one ID proof attached. Komathimuthupuram Post, Panaiyoor(via) Changarankovil Taluk, Tirunelveli, 627 761. 11 AP-64 S. Arun kannan No. 15D, Poonga Nagar, Kaladipet, Only one ID proof attached. Thiruvottiyur, Ch – 600 019 12 AP-69 K. Lavanya Priyadharshini No, 35, A Block, Nochi Nagar, Mylapore, Only one ID proof attached. Chennai – 600 004 13 AP-70 G. -
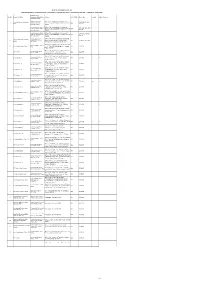
Address STD CODE Phone No Fax No E-Mail Address 1 2 3 4 5 6 7 8 9
RIGHT TO INFORMATION ACT 2005 NAME AND ADDRESS OF ASSISTANT PUBLIC INFORMATION OFFICERS AND PUBLIC INFORMATION OFFICERS - COMMERCIAL TAXES DEPT. Assistant Public Sl.No. Name of the Office Information Officer/Public Address STD CODE Phone No Fax No E-Mail Address Information Officer Deputy Commissioner Office of the Commissioner of Commercial Commissioner of Commercial 28546944,28514656 1 (Special Cell) Public Taxes, Chepauk, Ezhilagam Complex, Chennai 044 Taxes Extn .29 Information Officer 600 005. Asst.Commissioner (General Office of the Commissioner of Commercial 28546944, 28514656 Services) Assistant Public Taxes, Chepauk, Ezhilagam Complex, Chennai 044 Extn .25 Information Officer 600 005. Asst.Commissioner (Public Office of the Commissioner of Commercial 28546944,28514656 Relations)/ Assistant Public Taxes, Chepauk, Ezhilagam Complex, Chennai 044 Extn .44 Information Officer 600 005. Personal Asst. to Joint Office of the Joint Commissioner (Commercial Joint Commissioner,Chennai( 2 Commissioner, Chennai rd 044 28295551, 28294396 North ) Taxes) Chennai (North) 3 Floor PAPJM (North)/ PIO Buildings, No. 1 Greams Road, Chennai -6 Office of the Assistant Commissioner (CT) Zone- Asst.Commissioner, Zone - I 3 Asst. Commissioner - Zone - I rd 044 28295695 -- -- /PIO 1 3 Floor PAPJM Buildings, No. 1 Greams Road, Chennai -6 Office of the Commercial Tax Officer, Harbour I Commercial Tax Officer, CTO, Harbour - I Assessment Circle, Dass India Tower 044 25248777 - - Harbour - I /PIO Building,No.3,2nd Lane Beach,Chennai-1 Office of the Commercial -

Sankarankovil Final Report Tirunelveli District, Tamil Nadu - 1
City Corporate cum Business Plan for Sankarankovil Final Report Tirunelveli District, Tamil Nadu - 1 - 1 PROJECT OVERVIEW 1.1 ASSIGNMENT BACKGROUND The World Bank has been a partner in urban reform program of Government of Tamil Nadu (GoTN) with engagement through Tamil Nadu Urban Development Project (TNUDP) - TNUDP-I, TNUDP-II and TNUDP-III (in progress). Towards taking forward the urban reform agenda, the GoTN is now implementing the TNUDP-III with focus on furthering the reforms initiated under TNUDP-II. The Tamil Nadu Urban Infrastructure Financial Services Limited (TNUIFSL), as a financial intermediary, intends to assist the Commissioner of Municipal Administration (CMA) in strengthening and improving the financial position of its Municipalities for effective capital investment management and urban service delivery. These towns possess a good potential for implementation of such financial reforms for which it is essential to formulate a City Corporate Cum Business Plan. The CMA has started the process of capacity building in Municipalities through this process to enhance the vision of the ULBs in growth of their towns. The TNUIFSL has appointed M/s. Community Consulting India Private Limited (CCI) to prepare City Corporate Cum Business Plan (CCBP) for Sankarankovil Municipality. 1.1.1 CITY CORPORATE PLAN A City Corporate Plan (CCP) is the ULB’s corporate strategy that presents both a vision of a desired future perspective for the city and the ULB’s organization, and mission statements on how the ULB, together with other stakeholders, intends to work towards achieving their long- term vision in the next ten years. A CCP translates mission into actions and actions into outcomes. -

Updtd-Excel List of Doctors-2020.Xlsx
State / UT wise List of Doctors / Institution, authorised to issue Compulsory Health Certificate (for Shri Amarnathji Yatra 2020) Tamil Nadu Resident Medical Officers of the Medical College Hospitals under the control of Director of Medical Education,Chennai, Tamil Nadu mentioned below have been authorised to issue Compulory Health Certificate for the pilgrims of Shri Amarnathji Yqatra 2020 S.No District District Hospital Name of the Residential Phone / Mobile Medical Officer 1 Chennai Rajiv Gandhi Govt. Gen. Dr.Thirunavukkarasu S.K 9445030800 Hospital, Chennai 2 Govt. Stanley Hospital, Dr. Ramesh .M 98417-36989 Chennai 3 Kilpauk Medical College Dr. S. Rajakumar S 98842-26062 Hospital, Chennai 4 Institute of Mental Dr.Sumathi.S (I/C) 9677093145 Health, Chennai. 5 ISO &Govt.Kasturbna Dr.Elangovan S V 9840716412 Gandhi Hospital for Women & Children Chenai 6 Institute of Obstetrics Dr.Fatima (I/C) 7845500129 and Gyanecology and Govt.Hospital for Women & Children Chenai 7 Govt.Royapeetah Dr.Ananda Pratap M 9840053614 Hospital, Chennai 8 Institute of ChildHealth, Dr.Venkatesan (I/C) 8825540529 & Hospital for Children,Chennai-8 9 RIO & Govt. Opthalmic Dr.Senthil B 9381041296 Hospital, Chennai-8 10 Chengalpattu Chengalpattu Medical Dr. Valliarasi (I/c) 9944337807 College & Hospital,, Chengalpattu 11 thanjavur Thanjavur Medical Dr. Selvam 9443866578 , 9789382751 College & Hospital. thanjavur 12 Madurai Goverment Rajaji Dr. Sreelatha A. 9994793321 Hospital, Madurai 13 Coimbatore Coimbatore Medical Dr.Soundravel R 9842246171 College & Hospital 14 Salem Govt. Mohan Dr. Rani 9443246286 Kumaramangalam Medical College Hospital, Salem 15 Tirunelveli Tirunelveli Medical Dr. Shyam Sunder Singh N 9965580770 College & Hospital 16 Trichy Mahatma Gandhi Dr.Chandran (I/C) 9043500045 Memorial & Hospital, Trichy 17 Tuticorin Thoothukudi Medical Dr.Silesh Jayamani 9865131079 College & Hospital, Thoothukudi 18 Kanya kumari Govt. -

Tirunelveli Sl.No
TIRUNELVELI SL.NO. APPLICATION NO NAME AND ADDRESS MUTHUKUMAR P, S/O.PETCHIMUTHU K, PLOT 66 KARPAGAM, 1 3250 NAGAR 7TH ST, KTC NAGAR, PALAYAMKOTTAI THIRUNELVELI-627011 VIJAYALAKSHMI.N, D/O NADARAJAN, 2 3251 24, RAJENDRANAGAR, PALAYAMCOTTAI, THIRUNELVELI-627002 THANGAMANI. C D/O.M CHINNAPPA, ARIYANAYAKIPURAM, 3 3252 VEERASIGAMANI, SANKARANKOIL TK- THIRUNELVELI GOVINDARAJ.S, S/O.P.S UDALAIMANI,, 9/32,A.EAST STREET,, 4 3253 VAIKKALPATTY,, METTUR,AMBAI TK THIRUNELVELI-627436 NAGESWARI.N, W/O.MANIKANDAN, DOOR NO. 13/106, AMBALAVANAPURAM 5 3254 PERIA THERU, VICKRAMASINGA - PURAM, AMBASAMUDRAM PO, THIRUNELVELI-627425 SAKILA.S, D/O.SENTHILVEL, 157/2, WEST STREET,, 6 3255 KUVALAIKANNI, SANKARANKOVIL TK., THIRUNELVELI KANAGARAJ.S, S/O.A.SUDALAIMUTHU, 16/A, AMMANKOVIL ST, 7 3256 MEENATCHIPURAM, PANPOZHI, TENKASI TK., THIRUNELVELI-627807 RAVINDRAN.N, S/O NALLA PERUMAL.K, CHIDAMBARAPURAM, 8 3257 KURUVIKULAM VIA, SANKARANKOIL TK. THIRUNELVELIPage 1 RAMESH AMARNATH.B, S/O.S.BALASUBRA- MANIAN, NO:9, 5TH STREET, 9 3258 STATE BANK COLONY, MELAGARAM, TENKASI TK, TIRUNELVELI-627818. JAIKUMAR.G.S S/O.M.G.G.SEKAR, 18, PUTHANERI WEST, 10 3259 SINGANERI PO, NANGUNERI TK., THIRUNELVELI-627108 VELMURUGESWARI.R, W/O AYYAPPAN, NO.10/1037.E SELVA VINAYAGAR PURAM, 11 3260 PAVOOR CHATRAM, TENKASI THIRUNELVELI- 0 JANITA MANO C, W/O AMARAN S, MAIN ROAD, KUVALAI KANNI, 12 3261 KARIVALAM VANDANALLUR THIRUNELVELI 627753 GANAGAMMAL., 14,RASIPURAM, 13 3262 TIRUNELVELI TOWN, THIRUNELVELI 0 RAJESWARI.V D/O.P.VELUCHAMY, AMBEDKAR STREET, 14 3263 MEENATCHIPOURAM, PANPOZHI THIRUNELVELI 0 KUMAR.K, S./O.K.KARUPPIAH,, 13A, KARUPPASAMY 15 3264 KOVIL STREET, KRISHNAPURAM THIRUNELVELI-627811 Page 2 RAJESWARAN.P, S/O.M.PAULRAJ,, 5/76A, AMMAN KOVIL ST, 16 3265 ALAGAPPAPURAM,, SAMBANKULAM, AMBAI THIRUNELVELI-627412 ANBU RAJ. -

Lrc Centre – Tirunelveli
LEARNER RESOURCE CENTRE CENTRE SL.NO. DISTRICT NAME AND ADDRESS OF LRC CENTRES CODE Abacus Computer College Attoor Pulimoodu Jn., Thiruvattar Post – 629177 1 KANNIYAKUMARI 090501 Kanniyakumari District. Ph. 04651-283399, (m) 9442461395E-mail: [email protected] National Computer College 15-86/2, Court Road, Kuzhithurai – 629163, 2 KANNIYAKUMARI 090502 Kanyakumari District. Ph: 04651-264020 (M) 9443449201 E-mail: [email protected] WIIT College of Higher Education 14-58a, “Pavithiram Buildings”, Main Road, Mettukadai, 3 KANNIYAKUMARI 090503 Thuckalay-629 175. Kanniyakumari District 04651-253761. (M) 9443432804 email: [email protected] Gandhi Kamaraj Educational & Rural Development Trust (Opp.) Anna Bus StandUpstairs Indian Bank, 4 KANNIYAKUMARI 090504 Nagercoil-629001. Kanniyakumari District Ph. 04652-234748/ 325299 (M) 9345041515 email: [email protected] Master’s college of Higher Education 112/6, 1st Floor, Thomson Street, 5 KANNIYAKUMARI 090505 Nagarcoil – 1, Kanyakumari Dist (M) 9443449069, 9789329961 e-mail: [email protected] Yogi Kannya Educational Trust“Anugraha” No. 10 Parvathavarthini Street, Ramavarmapuram, 6 KANNIYAKUMARI 090506 Nagercoil – 629001.Kanyakumari District Ph: 04652-230799, (M) 9442006543 E-mail : [email protected] Boothalingam Educational & Social servic e Trust No.6/7E, R.K. Complex, Opp.Srinarayana Hospital, 7 KANNIYAKUMARI 090507 R.C.Street, Kaliakkavillai, Kanyakumari District – 629 153 Ph: 04651 - 245890 (M) 09388321758 / 9446326321 e-mail: [email protected] Kovilpatti Study -

Tamil Nadu Government Gazette
© [Regd. No. TN/CCN/467/2012-14. GOVERNMENT OF TAMIL NADU [R. Dis. No. 197/2009. 2014 [Price: Rs.330.40 Paise. TAMIL NADU GOVERNMENT GAZETTE PUBLISHED BY AUTHORITY No. 34] CHENNAI, WEDNESDAY, SEPTEMBER 3, 2014 Aavani 18, Jaya, Thiruvalluvar Aandu – 2045 Part VI—Section 4 Advertisements by private individuals and private institutions CONTENTS PRIVATE ADVERTISEMENTS Pages Change of Names .. 12649-2723 Notice .. 2724 NOTICE NO LEGAL RESPONSIBILITY IS ACCEPTED FOR THE PUBLICATION OF ADVERTISEMENTS REGARDING CHANGE OF NAME IN THE TAMIL NADU GOVERNMENT GAZETTE. PERSONS NOTIFYING THE CHANGES WILL REMAIN SOLELY RESPONSIBLE FOR THE LEGAL CONSEQUENCES AND ALSO FOR ANY OTHER MISREPRESENTATION, ETC. (By Order) Director of Stationery and Printing. CHANGE OF NAMES 39138. I, S. Raamesh, son of Thiru M. Santhanam, 39141. My son, Geeva born on 25th August 1999 born on 13th June 1991 (native district: Madurai), (native district: Dindigul), residing at No. 2/96, residing at No. 11-A, Anna Nagar Street, Madurai-625 002, Vakkampatty, Dindigul-624 002, shall henceforth be shall henceforth be known as S. RAMESH. known as T. JEEVAMANI. S. RAAMESH. M. F¼ŠðF. Madurai, 25th August 2014. Dindigul, 25th August 2014. (Father.) 39139. I, P. Joykrubha, daughter of Thiru K. John, 39142. I, R. Gayathri, daughter of Thiru A. Raja Gopal, born on 10th July 1993 (native district: Madurai), residing at born on 9th November 1993 (native district: Madurai), No. 2-1-75, T. Kallupatti, Peraiyur Taluk, Madurai-625 702, residing at No. 5/294, Vaigai Nathi Street, Magarajan Nagar, Viraganoor Post, Madurai-625 009, shall henceforth be shall henceforth be known as J. -

Tamil Nadu Government Gazette Published by Authority
© [Regd. No. TN/CCN/467/2012-14. GOVERNMENT OF TAMIL NADU [R. Dis. No. 197/2009. 2018 [Price : Rs. 4.80 Paise. TAMIL NADU GOVERNMENT GAZETTE PUBLISHED BY AUTHORITY No. 52] CHENNAI, WEDNESDAY, DECEMBER 26, 2018 Margazhi 11, Vilambi, Thiruvalluvar Aandu – 2049 Part VI—Section 1 Notifications of interest to the General Public issued by Heads of Departments, Etc. NOTIFICations BY HEADS OF Departments, ETC. CONTENTS Pages. GENERAL NOTIFICationS Winding up of the affairs of the IND. No. 567, South Arcot Police Forces Women Industrial Co-operative Society Ltd., and Appointment of Official Liquidator .. .. .. .. .. 398 Final Closing and Cancellation of Registration of TNH. 25, Sankarankovil Sontha Kaithari Weavers Co-operative Production and Sales Society Ltd., Sankarankovil, Sankarankovil Taluk, Tirunelveli District. 398 Winding up of the affairs of certain Co-operative Societies in certain Districts and appointment of Official Liquidator. O.995 in Sawyerpuram Senthiambalam Primary Weavers Co-operative Production and Sale Society Limited in Paramankurichi, Tiruchendur Taluk, Thoothukudi District .. .. .. .. .. .. 398 TNH.52 Ettayapuram Muppidariyamman Primary Weavers Co-operative Production and Sale Society Limited., in Paramankurichi, Tiruchendur Taluk, Thoothukudi District. .. .. .. .. .. 398 Variation to the Sanctioned High Ground Area Town Planning Scheme in V.M. Chathiram Village, Tirunelveli Local Planning Area .. .. .. .. .. .. .. .. 399 Variation to the Approved Courtallam Detailed Development Plan No. 1 of Courtallam Local Planning Area 399 Confirmation of Variation to the Approved Detailed Development Plan No. 10 of Vellore Local Planning Area. 403-404 Variation to the Approved Master Plan for the Coimbatore Local Planning Area. 404 Confirmation of Variation to the Approved Periyakanganankuppam Detailed Development Plan No. 1 of Cuddalore Local Planning Area. -

Count of Members by Females & Males in Clubs
GN1569 COUNT OF MEMBERS BY FEMALES & MALES IN CLUBS Figures Reflect Changes Reported on the January 2007 Club District Number Club Name Females Male TOTAL District 324B4 26466 ARUPPUKOTTAI 0 15 15 District 324B4 26487 KULASEKHARAM 0 28 28 District 324B4 26492 NAGERCOIL 1 27 28 District 324B4 26498 RAJAPALAYAM 0 85 85 District 324B4 26501 SAHUPURAM 2 42 44 District 324B4 26504 SIVAKASI 56 132 188 District 324B4 26507 TIRUNELVELI 2 37 39 District 324B4 26510 TUTICORIN 1 87 88 District 324B4 26512 VIRUDHUNAGAR 2 98 100 District 324B4 29707 SPICNAGAR 0 31 31 District 324B4 30405 PALAYAMKOTTAI 0 34 34 District 324B4 30844 SRIVILLIPUTHUR 0 29 29 District 324B4 31833 AMBASAMUDRAM 3 32 35 District 324B4 32156 PARAMAKUDI 2 66 68 District 324B4 39100 TENKASI 1 20 21 District 324B4 40829 WATRAP 0 23 23 District 324B4 43127 THIRUTHANGAL 0 36 36 District 324B4 43432 SATHUR 0 26 26 District 324B4 43948 ALANGULAM 0 44 44 District 324B4 43950 SHENCOTTAH 0 21 21 District 324B4 45243 KOVILPATTI MID TOWN 2 36 38 District 324B4 45624 THUCKALAY 0 26 26 District 324B4 49095 TUTICORIN CHIDAMBARANAR 1 20 21 District 324B4 49425 NEYYOOR MONDAY MARKET 0 18 18 District 324B4 49550 RAMANATHAPURAM SETHUPATHY 1 38 39 District 324B4 50048 KARINGAL 0 31 31 District 324B4 50049 NAGERCOIL TOWN 0 21 21 District 324B4 50050 TIRUCHENDUR 0 16 16 District 324B4 50051 TUTICORIN BHARATHY 4 35 39 District 324B4 50166 COLACHEL 0 21 21 District 324B4 50307 VALLIOOR 0 31 31 District 324B4 50398 ARUMANAI 3 13 16 District 324B4 50400 KILAKARAI SEETHAKATHI 0 31 31 District 324B4 51317 -
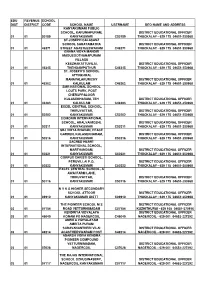
Deo &Ceo Address List
EDU REVENUE SCHOOL DIST DISTRICT CODE SCHOOL NAME USERNAME DEO NAME AND ADDRESS KANYAKUMARI PUBLIC SCHOOL, KARUNIAPURAM, DISTRICT EDUCATIONAL OFFICER 01 01 50189 KANYAKUMARI C50189 THUCKALAY - 629 175 04651-250968 ST.JOSEPH CALASANZ SCHOOL SAHAYAMATHA DISTRICT EDUCATIONAL OFFICER 01 01 46271 STREET AGASTEESWARAM C46271 THUCKALAY - 629 175 04651-250968 GNANA VIDYA MANDIR MADUSOOTHANAPURAM VILLAGE KEEZHAKATTUVILAI, DISTRICT EDUCATIONAL OFFICER 01 01 46345 THENGAMPUTHUR C46345 THUCKALAY - 629 175 04651-250968 ST. JOSEPH'S SCHOOL ATTINKARAI, MANAVALAKURICHY DISTRICT EDUCATIONAL OFFICER 01 01 46362 KALKULAM C46362 THUCKALAY - 629 175 04651-250968 SMR NATIONAL SCHOOL LOUTS PARK, POST CHERUPPALOOR KULASEKHARAM, TEH DISTRICT EDUCATIONAL OFFICER 01 01 46383 KALKULAM C46383 THUCKALAY - 629 175 04651-250968 EXCEL CENTRAL SCHOOL, THIRUVATTAR, DISTRICT EDUCATIONAL OFFICER 01 01 50202 KANYAKUMARI C50202 THUCKALAY - 629 175 04651-250968 COMORIN INTERNATIONAL SCHOOL, ARALVAIMOZHI, DISTRICT EDUCATIONAL OFFICER 01 01 50211 KANYAKUMARI C50211 THUCKALAY - 629 175 04651-250968 SBJ VIDYA BHAVAN, PEACE GARDEN, KULASEKHARAM, DISTRICT EDUCATIONAL OFFICER 01 01 50216 KANYAKUMARI C50216 THUCKALAY - 629 175 04651-250968 SACRED HEART INTERNATIONAL SCHOOL, MARTHANDAM, DISTRICT EDUCATIONAL OFFICER 01 01 50221 KANYAKUMARI C50221 THUCKALAY - 629 175 04651-250968 CORPUS CHRISTI SCHOOL, PERUVILLAI P.O, DISTRICT EDUCATIONAL OFFICER 01 01 50222 KANYAKUMARI C50222 THUCKALAY - 629 175 04651-250968 EXCEL CENTRAL SCHOOL, A AWAI FARM LANE, THIRUVATTAR, DISTRICT EDUCATIONAL OFFICER