Mechanical and Cytocompatibility Evaluation of UHMWPE/PCL/Bioglass® Fibrous Composite for Acetabular Labrum Implant
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
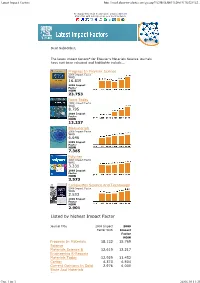
Listed by Highest Impact Factor
LatestImpactFactors http://mail.elsevieralerts.com/go.asp?%2fbESJ001%2fxFV7O22F%2... To display this email in a browser, please click here Share this with colleagues on your social network Dear Subscriber, The latest impact factors* for Elsevier's Materials Science journals have just been released and highlights include... Progress In Polymer Science 2008 Impact Factor WAS 16.819 2009 Impact Factor NOW 23.753 Nano Today 2008 Impact Factor WAS 8.795 2009 Impact Factor NOW 13.237 Biomaterials 2008 Impact Factor WAS 6.646 2009 Impact Factor NOW 7.365 Polymer 2008 Impact Factor WAS 3.331 2009 Impact Factor NOW 3.573 Composites Science And Technology 2008 Impact Factor WAS 2.533 2009 Impact Factor NOW 2.901 Listed by highest Impact Factor Journal Title 2008 Impact 2009 Factor WAS Impact Factor NOW Progress In Materials 18.132 15.769 Science Materials Science & 12.619 12.217 Engineering R-Reports Materials Today 12.929 11.452 Carbon 4.373 4.504 Current Opinions In Solid 2.976 4.000 State And Materials Science Стр.1из3 24.06.1011:25 LatestImpactFactors http://mail.elsevieralerts.com/go.asp?%2fbESJ001%2fxFV7O22F%2... Science Acta Biomaterialia: 3.727 3.975 Structure - Property - Function Relationships Acta Materialia 3.729 3.760 Scripta Materialia 2.887 2.949 Reactive & Functional 2.039 2.461 Polymers Composites Part 1.951 2.410 A-Applied Science And Manufacturing Cement And Concrete 1.549 2.376 Research Corrosion Science 2.293 2.316 European Polymer Journal 2.143 2.310 Intermetallics 2.034 2.231 Polymer Degradation And 2.320 2.154 Stability Journal -

Electrospun Thymosin Beta-4 Loaded PLGA/PLA Nanofiber/ Microfiber Hybrid Arnsy for Tendon Tissue Engineering Application
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Mechanical & Materials Engineering Faculty Mechanical & Materials Engineering, Publications Department of 2020 Electrospun Thymosin Beta-4 Loaded PLGA/PLA Nanofiber/ Microfiber Hybrid arnsY for Tendon Tissue Engineering Application Shaohua Wu University of Nebraska Medical Center Rong Zhou Qingdao University Fang Zhou Qingdao University Philipp N. Streubel University of Nebraska Medical Center, [email protected] Shaojuan Chen Qingdao University, [email protected] See next page for additional authors Follow this and additional works at: https://digitalcommons.unl.edu/mechengfacpub Part of the Mechanics of Materials Commons, Nanoscience and Nanotechnology Commons, Other Engineering Science and Materials Commons, and the Other Mechanical Engineering Commons Wu, Shaohua; Zhou, Rong; Zhou, Fang; Streubel, Philipp N.; Chen, Shaojuan; and Duan, Bin, "Electrospun Thymosin Beta-4 Loaded PLGA/PLA Nanofiber/ Microfiber Hybrid arnsY for Tendon Tissue Engineering Application" (2020). Mechanical & Materials Engineering Faculty Publications. 478. https://digitalcommons.unl.edu/mechengfacpub/478 This Article is brought to you for free and open access by the Mechanical & Materials Engineering, Department of at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Mechanical & Materials Engineering Faculty Publications by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Authors Shaohua Wu, Rong Zhou, Fang Zhou, Philipp N. Streubel, Shaojuan Chen, and Bin Duan This article is available at DigitalCommons@University of Nebraska - Lincoln: https://digitalcommons.unl.edu/ mechengfacpub/478 HHS Public Access Author manuscript Author ManuscriptAuthor Manuscript Author Mater Sci Manuscript Author Eng C Mater Manuscript Author Biol Appl. Author manuscript; available in PMC 2020 March 09. Published in final edited form as: Mater Sci Eng C Mater Biol Appl. -

Surface Characterization and in Vivo Evaluation of Laser Sintered and Machined Implants Followed by Resorbable-Blasting Media Process: a Study in Sheep
Med Oral Patol Oral Cir Bucal. 2016 Mar 1;21 (2):e206-13. Histology evaluation of laser sintered and machined implants Journal section: Oral Surgery doi:10.4317/medoral.20946 Publication Types: Research http://dx.doi.org/doi:10.4317/medoral.20946 Surface characterization and in vivo evaluation of laser sintered and machined implants followed by resorbable-blasting media process: A study in sheep Michelle Bowers 1, Daniel Yoo 1, Charles Marin 5, Luiz Gil 2, Nour Shabaka 3, Matt Goldstein 1, Malvin Janal 4, Nick Tovar 1, Ronaldo Hirata 1, Estevam Bonfante 1, Paulo Coelho 1 1 Department of Biomaterials and Biomimetics, New York University College of Dentistry, New York, USA 2 Department of Oral Surgery, Federal University of Santa Catarina, Florianópolis SC, Brazil 3 New York University Abu Dhabi, Abu Dhabi, United Arab Emirates 4 Department of Epidemiology, New York University College of Dentistry, New York, USA 5 Posgraduated program in Dentistry - Unigranrio University, School of Health Sciences, Duque de Caxias RJ, Brazil Correspondence: 345 E 24th Street 10010. Biomaterials and Biomimetics New York University New York, NY, USA Bowers M, Yoo D, Marin C, Gil L, Shabaka N, Goldstein M, Janal M, [email protected] Tovar N, Hirata R, Bonfante E, Coelho P. Surface characterization and in vivo evaluation of laser sintered and machined implants followed by resorbable-blasting media process: A study in sheep. Med Oral Patol Oral Cir Bucal. 2016 Mar 1;21 (2):e206-13. Received: 17/07/2015 http://www.medicinaoral.com/medoralfree01/v21i2/medoralv21i2p206.pdf Accepted: 14/10/2015 Article Number: 20946 http://www.medicinaoral.com/ © Medicina Oral S. -

2018 Journal Citation Reports Journals in the 2018 Release of JCR 2 Journals in the 2018 Release of JCR
2018 Journal Citation Reports Journals in the 2018 release of JCR 2 Journals in the 2018 release of JCR Abbreviated Title Full Title Country/Region SCIE SSCI 2D MATER 2D MATERIALS England ✓ 3 BIOTECH 3 BIOTECH Germany ✓ 3D PRINT ADDIT MANUF 3D PRINTING AND ADDITIVE MANUFACTURING United States ✓ 4OR-A QUARTERLY JOURNAL OF 4OR-Q J OPER RES OPERATIONS RESEARCH Germany ✓ AAPG BULL AAPG BULLETIN United States ✓ AAPS J AAPS JOURNAL United States ✓ AAPS PHARMSCITECH AAPS PHARMSCITECH United States ✓ AATCC J RES AATCC JOURNAL OF RESEARCH United States ✓ AATCC REV AATCC REVIEW United States ✓ ABACUS-A JOURNAL OF ACCOUNTING ABACUS FINANCE AND BUSINESS STUDIES Australia ✓ ABDOM IMAGING ABDOMINAL IMAGING United States ✓ ABDOM RADIOL ABDOMINAL RADIOLOGY United States ✓ ABHANDLUNGEN AUS DEM MATHEMATISCHEN ABH MATH SEM HAMBURG SEMINAR DER UNIVERSITAT HAMBURG Germany ✓ ACADEMIA-REVISTA LATINOAMERICANA ACAD-REV LATINOAM AD DE ADMINISTRACION Colombia ✓ ACAD EMERG MED ACADEMIC EMERGENCY MEDICINE United States ✓ ACAD MED ACADEMIC MEDICINE United States ✓ ACAD PEDIATR ACADEMIC PEDIATRICS United States ✓ ACAD PSYCHIATR ACADEMIC PSYCHIATRY United States ✓ ACAD RADIOL ACADEMIC RADIOLOGY United States ✓ ACAD MANAG ANN ACADEMY OF MANAGEMENT ANNALS United States ✓ ACAD MANAGE J ACADEMY OF MANAGEMENT JOURNAL United States ✓ ACAD MANAG LEARN EDU ACADEMY OF MANAGEMENT LEARNING & EDUCATION United States ✓ ACAD MANAGE PERSPECT ACADEMY OF MANAGEMENT PERSPECTIVES United States ✓ ACAD MANAGE REV ACADEMY OF MANAGEMENT REVIEW United States ✓ ACAROLOGIA ACAROLOGIA France ✓ -

Liste Des Revues SCOPUS
Liste des revues SCOPUS ENGINEERING Open N° Titre ISSN E-ISSN Acces Publisher Country Loc status 1 A + U-Architecture and Urbanism 03899160 Not OA Japan Architects Co., Ltd. Japan Orhan Hacihasanoglu ITU Faculty of 2 A|Z ITU Journal of Faculty of Architecture 13028324 Not OA Turkey Architecture A B B Corporate Management Services 3 ABB Review 10133119 Not OA Switzerland AG 4 ABU Technical Review 01266209 Not OA Asia-Pacific Broadcasting Union Malaysia 5 Accident Analysis and Prevention 00014575 Not OA Pergamon Press Ltd. United Kingdom 6 Accreditation and Quality Assurance 09491775 14320517 Not OA Springer Verlag Germany 7 ACI Materials Journal 0889325X Not OA American Concrete Institute United States 8 ACI Structural Journal 08893241 Not OA American Concrete Institute United States ACM Journal on Emerging Technologies in Association for Computing Machinary, 9 15504832 Not OA United States Computing Systems Inc. ACM Transactions on Autonomous and Association for Computing Machinary, 10 15564665 Not OA United States Adaptive Systems Inc. ACM Transactions on Design Automation of Association for Computing Machinary, 11 10844309 Not OA United States Electronic Systems Inc. ACM Transactions on Information and System Association for Computing Machinary, 12 10949224 Not OA United States Security Inc. 13 ACS Nano 19360851 1936086X Not OA American Chemical Society United States 14 ACS Synthetic Biology 21615063 Not OA American Chemical Society United States 15 Acta Astronautica 00945765 Not OA Pergamon Press Ltd. United Kingdom 16 Acta Biomaterialia -

Chemistry and Biochemistry Authored Faculty Publications
University of Arkansas, Fayetteville ScholarWorks@UARK University Libraries Faculty Publications and Presentations University Libraries 7-30-2020 Chemistry and Biochemistry Authored Faculty Publications: Snapshot of the Ranking of Journals in which they published based on data from Journal Citation Reports (JCR) Lutishoor Salisbury University of Arkansas, Fayetteville, [email protected] Yang Tian University of Arkansas, Fayetteville Jeremy Smith University of Arkansas, Fayetteville Follow this and additional works at: https://scholarworks.uark.edu/libpub Part of the Cataloging and Metadata Commons, Collection Development and Management Commons, Materials Chemistry Commons, and the Physical Chemistry Commons Citation Salisbury, L., Tian, Y., & Smith, J. (2020). Chemistry and Biochemistry Authored Faculty Publications: Snapshot of the Ranking of Journals in which they published based on data from Journal Citation Reports (JCR). University Libraries Faculty Publications and Presentations. Retrieved from https://scholarworks.uark.edu/libpub/38 This Report is brought to you for free and open access by the University Libraries at ScholarWorks@UARK. It has been accepted for inclusion in University Libraries Faculty Publications and Presentations by an authorized administrator of ScholarWorks@UARK. For more information, please contact [email protected]. University of Arkansas Libraries Chemistry and Biochemistry Library Chemistry and Biochemistry Authored Faculty Publications: Snapshot of the Ranking of Journals in which they published based on data from Journal Citation Reports (JCR). (https://go.openathens.net/redirector/uark.edu?url=https%3A%2F%2Fjcr.clarivate.com This report provides a snapshot of the ranking of journals for CHBC faculty authored papers for the period: 2015-2019. JCR is a database produced by Clarivate Analytics that provides impact factors and rankings of over 9000 journals. -

Elsevier Subscription Agreement
No l-6078425490 CRM la.1.0 20i07 ELSEVIER SUBSCRIPTION AGREEMENT This agreement ("'Agreement") is entered into as of 9 April 2014 by and between the Regents of the 1 University of California on behalf of the California Digital Library, 415 20th Street, 4 h Floor, Oakland, CA 94612 USA (the "Subscriber"), and Elsevier B.V., Radarweg 29, I 043 NX Amsterdam, The Netherlands (''Elsevier"). The parties hereto agree as follows: SECTION 1. SUBSCRIPTION. I .1 Subscribed Products. Elsevier hereby grants to the Subscriber the non-exclusive, non-transferable right to access and use the products and services identified in Schedule I ("Subscribed Products") and provide the Subscribed Products to its Authorized Users (as defined herein) which rights are worldwide with respect to off campus remote access by Authorized Users and perpetual as described in detail on Schedule 1 regarding access to formerly subscribed titles and archival print copies (which rights survive the termination of this Agreement), all subject to the terms and conditions of this Agreement. 1.2 Authorized Users/Sites. Authorized Users are the full-time and part-time students, faculty, staff and researchers of the Subscriber and individuals who are independent contractors or are employed by independent contractors of the Subscriber affiliated with the Subscriber's locations listed on Schedule 2 (the "Sites") and individuals using computer terminals within facilities at the Sites permitted by the Subscriber to access the Subscribed Products for purposes of personal research, education -

Bibliometric Analysis of Ongoing Projects: Innovative Medicines Initiative Joint Undertaking (IMI)
Bibliometric analysis of ongoing projects: Innovative Medicines Initiative Joint Undertaking (IMI) IMI EXECUTIVE OFFICE Fourth report: April 2014 Prepared by Thomson Reuters on behalf of IMI JU Executive Office under a public procurement procedure Copyright IMI JU April 2014 Disclaimer/Legal Notice: This document and attachments have been prepared solely for the Innovative Medicines Initiative Joint Undertaking (IMI JU). All contents may not be re-used (in whatever form and by whatever medium) by any third party without prior permission of the IMI JU. 2 Copyright IMI JU April 2014 1 Executive Summary .......................................................................................................................... 5 2 Introduction ....................................................................................................................................... 9 2.1 Overview ................................................................................................................................. 9 2.2 Innovative Medicines Initiative Joint Undertaking (IMI) .......................................................... 9 2.3 Thomson Reuters ................................................................................................................... 9 2.4 Thomson Reuters Research Analytics ................................................................................. 10 2.5 Thomson Reuters Custom Analytics & Engineered Solutions ............................................. 10 2.6 Scope of this report .............................................................................................................. -

Journal List of Scopus.Xlsx
Sourcerecord id Source Title (CSA excl.) (Medline-sourced journals are indicated in Green). Print-ISSN Including Conference Proceedings available in the scopus.com Source Browse list 16400154734 A + U-Architecture and Urbanism 03899160 5700161051 A Contrario. Revue interdisciplinaire de sciences sociales 16607880 19600162043 A.M.A. American Journal of Diseases of Children 00968994 19400157806 A.M.A. archives of dermatology 00965359 19600162081 A.M.A. Archives of Dermatology and Syphilology 00965979 19400157807 A.M.A. archives of industrial health 05673933 19600162082 A.M.A. Archives of Industrial Hygiene and Occupational Medicine 00966703 19400157808 A.M.A. archives of internal medicine 08882479 19400158171 A.M.A. archives of neurology 03758540 19400157809 A.M.A. archives of neurology and psychiatry 00966886 19400157810 A.M.A. archives of ophthalmology 00966339 19400157811 A.M.A. archives of otolaryngology 00966894 19400157812 A.M.A. archives of pathology 00966711 19400157813 A.M.A. archives of surgery 00966908 5800207606 AAA, Arbeiten aus Anglistik und Amerikanistik 01715410 28033 AAC: Augmentative and Alternative Communication 07434618 50013 AACE International. Transactions of the Annual Meeting 15287106 19300156808 AACL Bioflux 18448143 4700152443 AACN Advanced Critical Care 15597768 26408 AACN clinical issues 10790713 51879 AACN clinical issues in critical care nursing 10467467 26729 AANA Journal 00946354 66438 AANNT journal / the American Association of Nephrology Nurses and Technicians 07441479 5100155055 AAO Journal 27096 AAOHN -

State of California Approved by The
AGREEMENT NUMBER AM. NO. 4482 MASTER AGREEMENT CONTRACTOR IDENTIFICATION NUMBER 0000002886 THIS AGREEMENT, made and entered into this 11th day of December 2013 in the State of California, by and between the Trustees of the California State University, which is the State of California acting in a higher education capacity, through its duly appointed and acting officer, hereinafter called CSU and CONTRACTOR’S NAME Elsevier B.V. , hereafter called Contractor, WITNESSETH: That the Contractor for and in consideration of the covenants, conditions, agreements, and stipulation of the University hereinafter expressed, does hereby agree to furnish to the University services and materials as follows: Contractor shall provide online access to ScienceDirect online, Complete Collection, and Cell Press Collection for participating CSU campus libraries in accordance with the attached Riders, which are hereby incorporated by reference and made a part of this Agreement. To the extent that any provision of any rider may conflict with this CSU Agreement or any riders herein, the order of precedence shall first be the CSU Agreement and documents in the order listed below: Rider A – CSU General Provisions for Data Service Acquisitions, consisting of four (4) pages, Rider B – Elsevier Subscription Agreement, consisting of fifty-two (52) pages, Rider C – Participating CSU Campuses, consisting of one (1) page, Rider D – CSU Campus IP Addresses, consisting of two (2) pages. The term of this Agreement shall be January 1, 2014 through December 31, 2016. Contractor shall report to Eddie Choy, Director of ATS Contract Management. The total amount to be expended under this Agreement shall not exceed Ten Million, One Hundred Thousand, Eight Hundred Four and 77/100 dollars ($10,100,804.77). -

Open Science Platform Products and Services Agreement
OPEN SCIENCE PLATFORM PRODUCTS AND SERVICES AGREEMENT This open science platform products and services agreement ("Agreement") is entered into as of 15th of May 2020 by and between SURFmarket B.V., with its principal offices located at Moreelsepark 48, 3511 EP, Utrecht, The Netherlands, (“SURFmarket”), a limited liability organization, authorized to enter into this Agreement on behalf of the institutions identified in Schedule 2 (the "Institutions") (all, collectively, the “Customer”), and Elsevier B.V., Radarweg 29, 1043 NX, Amsterdam, The Netherlands ("Elsevier"). WHEREAS, SURFmarket provides certain intermediary services on behalf of the Institutions and wishes to enter into this Agreement on behalf of the Institutions. WHEREAS, both parties are committed to reach 100% Open Access during the term of this Agreement. Elsevier commits to deliver the Publishing Services in accordance with the terms and conditions as set forth in Schedule 4. Elsevier and the Institutions aspire to closely collaborate on services to support the research community to meet the Dutch ambition towards Open Science. WHEREAS, both parties have identified three types of services to focus their collaboration on as described in the MoU of December 2019: i. Reading Services ii. Publishing Services iii. Professional Services for Research Intelligence and Workflow WHEREAS, SURFmarket on behalf of the Institutions wishes to obtain rights to publish in Elsevier’s Gold Open Access journals in accordance with the Publishing Service terms and conditions set forth in Schedule 4 hereto. WHEREAS, both parties agree upon the Collaboration Principles as provided for in Schedule 5 (“Professional Services”). WHEREAS, parties have agreed to explore the opportunities to develop services in accordance with the terms and conditions set forth in Schedule 5 hereto (“Professional Services”). -

Web of Science® Science Citation Index Expandedtm 2012 March Web of Science®
REUTERS/Morteza Nikoubazl SOURCE PUBLICATION LIST FOR WEB OF SCIENCE® SCIENCE CITATION INDEX EXPANDEDTM 2012 MARCH WEB OF SCIENCE® - TITLE ISSN E-ISSN COUNTRY PUBLISHER 4OR-A Quarterly Journal of Operations Research 1619-4500 1614-2411 GERMANY SPRINGER HEIDELBERG AAPG BULLETIN 0149-1423 UNITED STATES AMER ASSOC PETROLEUM GEOLOGIST AAPS Journal 1550-7416 1550-7416 UNITED STATES SPRINGER AAPS PHARMSCITECH 1530-9932 1530-9932 UNITED STATES SPRINGER AMER ASSOC TEXTILE CHEMISTS AATCC REVIEW 1532-8813 UNITED STATES COLORISTS Abstract and Applied Analysis 1085-3375 1687-0409 UNITED STATES HINDAWI PUBLISHING CORPORATION ABDOMINAL IMAGING 0942-8925 1432-0509 UNITED STATES SPRINGER ABHANDLUNGEN AUS DEM MATHEMATISCHEN SEMINAR DER 0025-5858 1865-8784 GERMANY SPRINGER HEIDELBERG UNIVERSITAT HAMBURG ABSTRACTS OF PAPERS OF THE AMERICAN CHEMICAL 0065-7727 UNITED STATES AMER CHEMICAL SOC SOCIETY Academic Pediatrics 1876-2859 1876-2867 UNITED STATES ELSEVIER SCIENCE INC Accountability in Research-Policies and Quality Assurance 0898-9621 1545-5815 UNITED STATES TAYLOR & FRANCIS LTD Acoustics Australia 0814-6039 AUSTRALIA AUSTRALIAN ACOUSTICAL SOC UNIV CHILE, CENTRO INTERDISCIPLINARIO Acta Bioethica 0717-5906 1726-569X CHILE ESTUDIOS BIOETICA Acta Biomaterialia 1742-7061 1878-7568 ENGLAND ELSEVIER SCI LTD Acta Botanica Brasilica 0102-3306 1677-941X BRAZIL SOC BOTANICA BRASIL Acta Botanica Mexicana 0187-7151 MEXICO INST ECOLOGIA AC Acta Cardiologica Sinica 1011-6842 TAIWAN TAIWAN SOC CARDIOLOGY Acta Chirurgiae Orthopaedicae et Traumatologiae Cechoslovaca