CAPITOL REEF NATIONAL PARK, UTAH by David George Christiana
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

West Colorado River Plan
Section 9 - West Colorado River Basin Water Planning and Development 9.1 Introduction 9-1 9.2 Background 9-1 9.3 Water Resources Problems 9-7 9.4 Water Resources Demands and Needs 9-7 9.5 Water Development and Management Alternatives 9-13 9.6 Projected Water Depletions 9-18 9.7 Policy Issues and Recommendations 9-19 Figures 9-1 Price-San Rafael Salinity Control Project Map 9-6 9-2 Wilderness Lands 9-11 9-3 Potential Reservoir Sites 9-16 9-4 Gunnison Butte Mutual Irrigation Project 9-20 9-5 Bryce Valley 9-22 Tables 9-1 Board of Water Resources Development Projects 9-3 9-2 Salinity Control Project Approved Costs 9-7 9-3 Wilderness Lands 9-8 9-4 Current and Projected Culinary Water Use 9-12 9-5 Current and Projected Secondary Water Use 9-12 9-6 Current and Projected Agricultural Water Use 9-13 9-7 Summary of Current and Projected Water Demands 9-14 9-8 Historical Reservoir Site Investigations 9-17 Section 9 West Colorado River Basin - Utah State Water Plan Water Planning and Development 9.1 Introduction The coordination and cooperation of all This section describes the major existing water development projects and proposed water planning water-related government agencies, and development activities in the West Colorado local organizations and individual River Basin. The existing water supplies are vital to water users will be required as the the existence of the local communities while also basin tries to meet its future water providing aesthetic and environmental values. -

Geology and Stratigraphy Column
Capitol Reef National Park National Park Service U.S. Department of the Interior Geology “Geology knows no such word as forever.” —Wallace Stegner Capitol Reef National Park’s geologic story reveals a nearly complete set of Mesozoic-era sedimentary layers. For 200 million years, rock layers formed at or near sea level. About 75-35 million years ago tectonic forces uplifted them, forming the Waterpocket Fold. Forces of erosion have been sculpting this spectacular landscape ever since. Deposition If you could travel in time and visit Capitol Visiting Capitol Reef 180 million years ago, Reef 245 million years ago, you would not when the Navajo Sandstone was deposited, recognize the landscape. Imagine a coastal you would have been surrounded by a giant park, with beaches and tidal flats; the water sand sea, the largest in Earth’s history. In this moves in and out gently, shaping ripple marks hot, dry climate, wind blew over sand dunes, in the wet sand. This is the environment creating large, sweeping crossbeds now in which the sediments of the Moenkopi preserved in the sandstone of Capitol Dome Formation were deposited. and Fern’s Nipple. Now jump ahead 20 million years, to 225 All the sedimentary rock layers were laid million years ago. The tidal flats are gone and down at or near sea level. Younger layers were the climate supports a tropical jungle, filled deposited on top of older layers. The Moenkopi with swamps, primitive trees, and giant ferns. is the oldest layer visible from the visitor center, The water is stagnant and a humid breeze with the younger Chinle Formation above it. -

Our Secret Is Now Yours!
EXPLORE... THREE ADVENTUROUS ZONES Travel Guide Travel The Highlands Capitol Reef East Desert Our secret is now yours! Wayne County, - Utah - USA TABLE OF CONTENTS WELCOME Wayne County, Utah - Capitol Reef Country 1- Cover Calendar of Events 2- Table of Contents April to October - Daily Walks / Capitol Reef Welcome to the scenically diverse region encompassing Wayne County YOUR NEXT GREAT 3- Welcome May Utah’s Capitol Reef Country. - Entrada Institute’s Cowboy Poetry and Music DESTINATION 4- Zones Festival This vacation destination includes mountains, forests, pinnacles, arches, 5- Zones July plateaus, and astounding desert - Torrey Apple Days landscapes. The contrast in elevation 6- Attractions - Capitol Reef Classic – Annual High Desert Bike and terrain makes this area a haven Race. for those seeking solitude or - Bicknell International Film Festival (BIFF) 7- Attractions outdoor adventure. August This region is an excellent multi-day 8- Activities - Women’s Redrock Music Festival vacation destination, central to Utah’s - Wayne County Fair scenic attractions, and easily acces- 9- Guides & Outfitters sible from major cities such as Salt September/October Lake City, Denver, and Las Vegas. 10- Maps - Heritage Star Festival - Wayne Wonderland Air show Unpack once and discover a realm 11- Maps that is conveniently situated be- On Going Events: May - September tween Bryce Canyon, the Grand 12- Hotels / Motels Staircase, Goblin Valley State Park, Capitol Reef National Park: Daily walks and nightly Canyonlands National Park, and amphitheatre programs mid May - September. Lake Powell. 13- Cabins / B&B Check at Visitor Center for Ripple Rock Nature Center schedule. Capitol Reef National Park. You’ll want to reserve several days to 14- Campgrounds (435) 425-3791, ext. -

Episodic Sediment Delivery and Landscape Connectivity in the Mancos Shale Badlands and Fremont River System, Utah, USA
Geomorphology 102 (2008) 242–251 Contents lists available at ScienceDirect Geomorphology journal homepage: www.elsevier.com/locate/geomorph Episodic sediment delivery and landscape connectivity in the Mancos Shale badlands and Fremont River system, Utah, USA Andrew E. Godfrey 1, Benjamin L. Everitt a, José F. Martín Duque b,⁎ a 170 W. 300 North, Ivins, Utah 84738, USA b Department of Geodynamics, Complutense University, 28040 Madrid, Spain ARTICLE INFO ABSTRACT Article history: The Fremont River drains about 1000 km2 of Mancos Shale badlands, which provide a large percentage of the Accepted 23 May 2007 total sediment load of its middle and lower reaches. Factors controlling sediment movement include: Available online 18 May 2008 weathering that produces thin paralithic soils, mass movement events that move the soil onto locations susceptible to fluvial transport, intense precipitation events that move the sediment along rills and across Keywords: local pediments, and finally Fremont River floods that move the sediment to the main-stem Colorado River. Mancos Shale badlands A forty-year erosion-pin study has shown that down-slope creep moves the weathered shale crust an Erosion Mass movement average of 5.9 cm/yr. Weather records and our monitoring show that wet winters add large slab failures and Connectivity mudflows. Recent sediment-trap studies show that about 95% of sediment movement across pediments is Coupling accomplished by high-intensity summer convective storms. Between 1890 and 1910, a series of large autumn Arroyo cutting floods swept down the Fremont River, eroding its floodplain and transforming it from a narrow and meandering channel to a broad, braided one. -
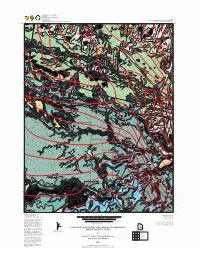
Geologic Map of the Twin Rocks Quadrangle
UTAH GEOLOGICAL SURVEY a division of Utah Department of Natural Resources in cooperation with Plate 1 National Park Service Utah Geological Survey Miscellaneous Publication 07-3 and Brigham Young University Department of Geological Sciences Geologic Map of the Twin Rocks Quadrangle Qe JTRw Qal Qmt Jno Jno Qms Jn JTRw Jno Jk Jn Qe Qe Jn Jn Jpc Jk Jpcr Qe Qmt Qe Jno Jno Jno JTRw Jno Jk Qe Qe Jn Qms Jn Jno Jno Jno Jno Ti 4200 JTRw Jno Jno 4600 4000 Jk Jno W A T E R P O C K E T Jn Qmt Qe Jn Ti Jno Qe 5000 Jno Jn Ti Qms Qms Qms Qmt Qmt Qmt 4400 Jk Jn JTRw Jn Qe Jno Qms Jno Jn Qe Qe Jn Qe Jn Jn Ti Jno Jno Jno Jn Jno Jn Jn Jk JTRw Qe Qmt Jk Jno TRco Qmt JTRw Jno TRco Qmt Jn Jno Qe Qmt TRcm TRco Qmt JTRw Jno Jno TRcp TRcp Jno Qmt Qal Jk Ti Qal Qmt JTRw Jk TRcp TRcp Jno Jn Jn TRcm Qmt Jno TRco Jno 4800 Qmt TRcp TRco Qal C A P I T O L R E E F Jno TRco F O L D Qmt JTRw Jno Qe Jno TRcp Qmt Jn JTRw JTRw Spring Jk Jno JTRw Qmt Jno Jk Jk Qmt Qmt TRco 5 Jn TRco Ti Qmt Qmt Jno Jk Qmt JTRw Canyon R TRco Qmt T co Qe JTRw Qmt R Qe TRco TRco TRco Tco Ti Qmt Qal C A P I T O L Qmt TRco TRco Jk TRco 5800 JTRw Qal JTRw Jno Jk Jno Jno Qmt TRco Qe Qe JTRw Jk Qe Jn Jno Jno Ti Jn JTRw Qmt Jk Jk Jno Jno Jno Jn JTRw Jk 5000 Jno JTRw Jno 4 Qe Qe R A' T co Qmt 5400 Jk Jn Jno 5600 Qe Qe Jno TRcp Qe Jn TRcm Qe Qmt Jno Jno JTRw Qe M E E K S Jn Ti Qe 3 4800 Jno Qe R E E F Jno Jno Qe Qal JTRw Qe Qe Jno Jn Qmt Qe Qe Jk Jk R Jk Jno Jno JT w Qmt Jno Jno TRco M E S A Qmt Jno Jno Qmt Qal Jno Qe Qe Qe Qmt Jk Jn Qmt TRco Qe TRco Jno Jk Qmt Qe Qal JTRw JTRw Jk TRco TRcp TRcp 5200 -

Geologic Resource Evaluation Report, Capitol Reef National Park
National Park Service U.S. Department of the Interior Natural Resource Program Center Capitol Reef National Park Geologic Resource Evaluation Report Natural Resource Report NPS/NRPC/GRD/NRR—2006/005 Capitol Reef National Park Geologic Resource Evaluation Report Natural Resource Report NPS/NRPC/GRD/NRR—2006/005 Geologic Resources Division Natural Resource Program Center P.O. Box 25287 Denver, Colorado 80225 September 2006 U.S. Department of the Interior Washington, D.C. The Natural Resource Publication series addresses natural resource topics that are of interest and applicability to a broad readership in the National Park Service and to others in the management of natural resources, including the scientific community, the public, and the NPS conservation and environmental constituencies. Manuscripts are peer-reviewed to ensure that the information is scientifically credible, technically accurate, appropriately written for the intended audience, and is designed and published in a professional manner. Natural Resource Reports are the designated medium for disseminating high priority, current natural resource management information with managerial application. The series targets a general, diverse audience, and may contain NPS policy considerations or address sensitive issues of management applicability. Examples of the diverse array of reports published in this series include vital signs monitoring plans; "how to" resource management papers; proceedings of resource management workshops or conferences; annual reports of resource programs or divisions of the Natural Resource Program Center; resource action plans; fact sheets; and regularly-published newsletters. Views and conclusions in this report are those of the authors and do not necessarily reflect policies of the National Park Service. Mention of trade names or commercial products does not constitute endorsement or recommendation for use by the National Park Service. -

Fremont River Watershed Water Quality Management Plan
FFrreemmoonntt RRiivveerr WWaatteerrsshheedd Water Quality Management Plan September 27, 2002 Includes TMDLs for: Johnson Valley Reservoir Forsyth Reservoir Mill Meadow Reservoir UM Creek Upper Fremont River Lower Fremont River i FREMONT RIVER WATERSHED WATER QUALITY MANAGEMENT PLAN TABLE OF CONTENTS 1.0 INTRODUCTION .................................................................................................... 1 1.1 The TMDL Process ........................................................................................ 1 1.2 Utah’s Watershed Approach .......................................................................... 3 2.0 WATERSHED DESCRIPTION ............................................................................... 5 2.1 Historic Perspective ....................................................................................... 5 2.2 Climate........................................................................................................... 6 2.3 Geology / Soils............................................................................................... 8 2.4 Land Use / Land Cover ................................................................................ 11 2.5 Hydrology..................................................................................................... 12 2.5.1 Reservoirs ....................................................................................... 12 2.5.2 Subbasins ....................................................................................... 18 2.5.3 Irrigation -

Vascular Flora and Vegetation of Capitol Reef National Park, Utah
Vascular Flora and Vegetation of Capitol Reef National Park, Utah Kenneth D. Heil, J. Mark Porter, Rich Fleming, and William H. Romme Technical Report NPS/NAUCARE/NRTR-93/01 National Park Service Cooperative Park Studies Unit U.S. Department of the Interior at Northern Arizona University National Park Service Cooperative Park Studies Unit Northern Arizona University The National Park Service (NPS) Cooperative Park Studies Unit (CPSU) at Northern Arizona University (NAU) is unique in that it was conceptualized for operation on an ecosystem basis, rather than being restrained by state or NPS boundaries. The CPSU was established to provide research for the 33 NPS units located within the Colorado Plateau, an ecosystem that shares similar resources and their associated management problems. Utilizing the university's physical resources and faculty expertise, the CPSU facilitates multidisciplinary research in NPS units on the Colorado Plateau, which encompasses four states and three NPS regions—Rocky Mountain, Southwest, and Western. The CPSU provides scientific and technical guidance for effective management of natural and cultural resources within those NPS units. The National Park Service disseminates the results of biological, physical, and social science research through the Colorado Plateau Technical Report Series. Natural resources inventories and monitoring activities, scientific literature reviews, bibliographies, and proceedings of techni cal workshops and conferences are also disseminated through this series. Unit Staff Charles van Riper, III, Unit Leader Peter G. Rowlands, Research Scientist Henry E. McCutchen, Research Scientist Mark K. Sogge, Ecologist Charles Drost, Zoologist Elena T. Deshler, Biological Technician Paul R. Deshler, Technical Information Specialist Connie C. Cole, Editor Margaret Rasmussen, Administrative Clerk Jennifer Henderson, Secretary National Park Service Review Documents in this series contain information of a preliminary nature and are prepared primarily for internal use within the National Park Service. -

Utah Scenic Byways Guide
Utah is the place where prehistory intersects with the enduring spirit of the Old West. Wild, adventure-rich places cradle vibrant urban centers. With interstates and airplanes, the world can feel pretty small. On Utah’s designated scenic byways, the world feels grand; its horizons seem infinite. As you drive through Utah, you’ll inevitably encounter many of the state’s scenic byways. In total, Utah’s distinct topography provides the surface for 27 scenic byways, which add up to hundreds of miles of vivid travel experiences wherein the road trip is as memorable as the destination. Utah’s All-American Road: Scenic Byway 12 headlines the network of top roads thanks to landscapes and heritage unlike anywhere else in the nation. All of Utah’s scenic byways are explorative journeys filled with trailheads, scenic overlooks, museums, local flavors and vibrant communities where you can stop for the night or hook up your RV. Not sure where to start? In the following pages, you’ll discover monumental upheavals of exposed rock strata among multiple national and state parks along the All-American Road (pg. 4); dense concentrations of fossils along Dinosaur Diamond (pg. 8); and the blazing red cliffs and deep blue waters of Flaming Gorge–Uintas (pg. 12) — and that’s just in the first three highlighted byways. Your journey continues down two dozen additional byways, arranged north to south. Best of all, these byways access an outdoor adventureland you can hike, fish, bike, raft, climb and explore from sunup to sundown — then stay up to welcome the return of the Milky Way. -

Capitol Reef National Monument
THIS WATER-CARVED LAND Thereafter, the area was covered by a series of calm, land-locked basins in which were deposited Water has been responsible, in one form or an- the soft, reddish-brown Entrada and the hard. other, for the spectacular scenery you see before gray Curtis Sandstones. Also deposited in these you. Water, in the form of rivers carrying tremen- shallow lakes was the Summerville Formation. dous loads of stones, pebbles, and grit, ground with its thin even bedding. much like the earlier away at rocks and cliffs to carve the valleys. Moenkopi. The most accessible view of these Water, as glaciers, helped grind the rocks and formations is on the eastern edge of the monu- boulders into soil. Water, as ice, also created the ment near the turnoff to Notom. pressures which split rocks to start the processes that made the soil beneath your feet. Water, as Once again the land uplifted and created a wide rain, softened and eroded the hard materials and flood plain. then settled and became a swampy transported them to other areas. lake. During this time. the colorful Morrison Formation. with its reds. purples. green3, yellows, The geologic story goes back millions of years. and grays, was deposited. It can be seen best as Two deposits are of the Permian Period, when a you approach the monument from the east, where shallow sea covered the area before the begin- it forms badlands. nings of life, but these are not easily seen. How- ever, standing by the visitor center, you can see This tranquil setting of swamp deposition was several formations which lie above the Kaibab changed by the slow encroachment of the Cre- Formation of the Permian. -

A Hydrometeorological Look at a Utah Flash Flood
Western Region Technical Attachment No. 93-22 August 3, 1993 A HYDROMETEOROLOGICAL LOOK AT A UTAH FLASH FLOOD Brian Mcinerney · WSFO Salt Lake City Craig C. Schmidt · WSFO Portland Introduction On Friday, July 24, 1992, a flash flood occurred on the Grand Wash in south central Utah and continued into Capitol Reef National Park on the Fremont River (Map 1). July 24th is a state holiday, with peak visitor attendance in the state's national parks. During this period, the Grand Wash is normally dry and only experiences flows during very heavy runoff. On the morning of the flash flood, the wash was dry, but quickly rose and became a flash flood by early afternoon. Many visitors were swimming in the Fremont River at the park during the early afternoon hours, and left just minutes before the flooding would have reached them. Other recreationalists were stranded in and near the area due to the rising water in both the Grand Wash and the Fremont River. Meteorological Discussion The flash flood was caused by a single thunderstorm cell that developed in a typical synoptic weather pattern for July in Utah. A number of meteorological factors considered favorable for thunderstorm development existed, but flash flood potential was minimal. At 1200 UTC 24 July, a positive-tilt trough was present over the western United States, with the axis extending from the Washington/ Idaho border, across Oregon, and over the Pacific Ocean. At 250 mb (Fig. 1), the Great Basin was under a strong southwesterly jet ahead of the trough, with southern Utah positioned under the favorable right-rear quadrant of the jet maximum. -

Andrew E. Godfrey" Benjamin L. Everitt A, Jose F. Martin Duque B.*
Episodic sediment delivery and landscape connectivity in the Mancos Shale badlands and Fremont River system, Utah, USA Andrew E. Godfrey" Benjamin L. Everitt a, Jose F. Martin Duque b.* ·170 W 300 North, Ivins, Utah 84738, USA b Department of Geodynamics, Complutense UnivelSity, 28040 Madrid, Spain ABSTRACT The FremontRiver drains about 1000 km2 of Mancos Shale badlands, which provide a large percentage of the total sediment load of its middle and lower reaches. Factors controlling sediment movement include: weathering that produces thin paralithic soils, mass movement events that move the soil onto locations susceptible to fluvial transport, intense precipitation events that move the sediment along rills and across Keywords: local pediments, and finally FremontRiver floods that move the sediment to the main-stem ColoradoRiver. Mancos Shale badlands Erosion A forty-year erosion-pin study has shown that down-slope creep moves the weathered shale crust an Mass movement average of 5.9 cm/yr. Weather records and our monitoring show that wet winters add large slab failures and Connectivity mudflows.Recent sediment-trap studies show that about 95% of sediment movement across pediments is Coupling accomplished by high-intensity summer convective storms.Between 1890 and 1910, a series of large autumn Arroyo cutting floods swept down the Fremont River, eroding its floodplain and transforming it from a narrow and meandering channel to a broad, braided one.Beginning about 1940, the Fremont's channel began to narrow. Sequential aerial photos and cross-sections suggest that floodplain construction since about 1966 has stored about 4000 to 8000 m3 of sediment per kilometer per year.