SFU Library Thesis Template
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

1.0 Introduction
Skeena Watershed Conservation Coalition P.O. Box 70 Hazelton, B.C. V0J 1Y0 www.skeenawatershed.com 250.842.2494 March 10, 2014 Canadian Environmental Assessment Agency 410-701 Georgia Street West Vancouver, BC V7Y 1C6 Via email: [email protected] Honourable Catherine McKenna Minster of Environment and Climate Change Via email: [email protected] Honorable Hunter Tootoo Minister of Fisheries, Oceans and the Canadian Coast Guard Ottawa, Ontario <Original signed by> Via email: [email protected]. Honourable James Carr, Minister of Natural Resources Via email: [email protected] Pacific Northwest LNG Project – Public Comments re CEAA draft Environmental Assessment Report From: Skeena Watershed Conservation Coalition CEAA Reference: 80032 1.0 Introduction 1.1 Skeena Watershed Conservation Coalition Skeena Watershed Conservation Coalition (SWCC) is a diverse group of people living and working in the Skeena River watershed. Our board of directors and membership reflect the broad interests of the people in this region. We are united in understanding that short term industrial development plans, even 50 year plans, will not benefit our region in the long run if they undermine the social and environmental fabric that holds the watershed and its communities together. SWCC’s mission is to cultivate a sustainable future from a sustainable environment rooted in culture and a wild salmon ecosystem. Objectives and strategies arising from this 1 mission include educating the public and decision-makers in order to increase awareness and understanding of the natural ecological and human assets that currently exist, as well as helping to create a vibrant and resilient future for the Skeena watershed. -

Pacific Northwest LNG Project
Pacific NorthWest LNG Project Environmental Assessment Report September 2016 Cover photo credited to Pacific NorthWest LNG Ltd. © Her Majesty the Queen in Right of Canada, represented by the Minister of Environment and Climate Change Catalogue No: En106-136/2015E-PDF ISBN: 978-1-100-25630-6 This publication may be reproduced in whole or in part for non-commercial purposes, and in any format, without charge or further permission. Unless otherwise specified, you may not reproduce materials, in whole or in part, for the purpose of commercial redistribution without prior written permission from the Canadian Environmental Assessment Agency, Ottawa, Ontario K1A 0H3 or info@ceaa- acee.gc.ca This document has been issued in French under the title: Projet de gaz naturel liquéfié Pacific NorthWest - Rapport d’évaluation environnementale Executive Summary Pacific NorthWest LNG Limited Partnership (the proponent) is proposing the construction, operation, and decommissioning of a new facility for the liquefaction, storage, and export of liquefied natural gas (LNG). The Pacific NorthWest LNG Project (the Project) is proposed to be located primarily on federal lands and waters administered by the Prince Rupert Port Authority approximately 15 kilometres south of Prince Rupert, British Columbia. At full production, the facility would receive approximately 3.2 billion standard cubic feet per day, or 9.1 x 107 cubic metres per day, of pipeline grade natural gas, and produce up to 20.5 million tonnes per annum of LNG for over 30 years. The Project would include the construction and operation of a marine terminal for loading LNG on to vessels for export to Pacific Rim markets in Asia. -

Pacific North West LNG Project: a Review and Assessment of the Project Plans and Their Potential Impacts on Marine Fish and Fish Habitat in the Skeena Estuary
Pacific North West LNG Project: A review and assessment of the project plans and their potential impacts on marine fish and fish habitat in the Skeena estuary. Asit Mazumder Professor Department of Biology University of Victoria Victoria June 2016 1 Table of Contents I. Purpose ........................................................................................................................................................... 3 Methodology ..................................................................................................................................................................... 3 II. The Existing Environment ........................................................................................................................ 4 III. Project Design ............................................................................................................................................ 5 IV. Potential Effects on Marine Fish and ........................................................................ Fish Habitat 8 Deleted: 9 Marine Structure Impacts on Sediment Transport ish and F Habitat ........................................................ 10 V. Conceptual Fish Habitat Offsetting ................................................................................. Strategy 16 Deleted: 15 VI. CEAA Assessment of PNW-‐‑LNG Proposal on ............................................ Marine Resources 20 Deleted: 19 VII. Conclusions of the Review ................................................................................................................. -

The Environmental Implications of Sediment Transport in the Waters Of
Journal of Coastal Research 32 3 465–482 Coconut Creek, Florida May 2016 The Environmental Implications of Sediment Transport in the Waters of Prince Rupert, British Columbia, Canada: A Comparison Between Kinematic and Dynamic Approaches Patrick McLaren SedTrend Analysis Limited Brentwood Bay, BC V8M1C5, Canada [email protected] ABSTRACT McLaren, P., 2016. The environmental implications of sediment transport in the waters of Prince Rupert, British Columbia, Canada: A comparison between kinematic and dynamic approaches. Journal of Coastal Research, 32(3), 465– 482. Coconut Creek (Florida), ISSN 0749-0208. A Sediment Trend Analysis (STAt) was performed on 2474 grain-size distributions taken from the Port of Prince Rupert, British Columbia, Canada. The analysis was commissioned by the Lax Kw’alaams First Nations Band because of environmental concerns associated with future large-scale development plans, including a proposed liquefied natural gas (LNG) terminal associated with Flora Bank. Located at the mouth of the Skeena River, Flora Bank has long been considered an important nursery area for juvenile salmon. STA is an empirical technique to determine patterns of net sediment transport, which may provide a qualitative assessment of the possible environmental changes that could be expected following port construction. The patterns of transport revealed that sediments throughout the study area are derived from underlying till which is exposed in areas of strong currents. Flora Bank, a roughly 4 km2 area of intertidal sand, contained the coarsest and most well sorted sand, which was not found elsewhere throughout the study area. Although derived from till, the sand did not form transport pathways from the other sediment types; in addition, pathways could not be determined on the bank itself. -
Technical Data Report: Marine Resources
APPENDIX M – PART 1 APPENDIX M Technical Data Report - Marine Resources PACIFIC NORTHWEST LNG Technical Data Report – Marine Resources Prepared for: Prepared by: Pacific NorthWest LNG Limited Partnership Stantec Consulting Ltd. Oceanic Plaza, Suite 1900 - 1066 West Hastings Street 4370 Dominion Street, Suite 500 Vancouver, BC V6E 3X1 Burnaby, BC V5G 4L7 Tel: (778) 372-4700 | Fax: (604) 630-3181 Tel: (604) 436-3014 | Fax: (604) 436-3752 Project No.: Date: 1231-10537 February 17, 2014 Pacific NorthWest LNG Technical Data Report – Marine Resources Authorship AUTHORSHIP Philip Molloy, B.Sc., Ph.D. ................................................................................... Co-Lead Author Aimee Gromack, B.Sc., MMM ............................................................................. Co-Lead Author Conor McCracken, B.Sc., BIT .................................................................................... Contributor William Brewis, Dip., B.Sc., M.Sc. ............................................................................. Contributor Rowenna Gryba, B.Sc., M.Sc. ................................................................................... Contributor Marc Skinner, C.D., M.Sc... ........................................................................................ Contributor Heather Ward, B.Sc., M.Sc. ....................................................................................... Contributor Christine Gruman, B.Sc., MRM ................................................................................. -

The Skeena River Estuary
Morphodynamics of a Bedrock Confined Estuary and Delta: The Skeena River Estuary by Amanda Lily Wild B.Sc., University of Victoria, 2018 A Thesis Submitted in Partial Fulfillment of the Requirements for the Degree of Master of Science In the Department of Geography ©Amanda Wild, 2020 University of Victoria All rights reserved. This thesis may not be reproduced in whole or in part, by photocopy or other means, without the permission of the author. Morphodynamics of a Bedrock Confined Estuary and Delta: The Skeena River Estuary By Amanda Lily Wild B.Sc., University of Victoria, 2018 Supervisory Committee Dr. Eva Kwoll, Co-Supervisor Department of Geography Dr. Gwyn Lintern, Co-Supervisor Natural Resources Canada ii Abstract Bedrock islands add variation to the estuarine system that results in deviations from typical unconfined estuarine sediment transport patterns. Limited literature exists regarding the dynamics of seabed morphology, delta formation, sediment divergence patterns, and sedimentary facies classifications of non-fjordic bedrock confined systems. Such knowledge is critical to address coastal management concerns adequately. This research presents insights from the Skeena Estuary, a macrotidal estuary in northwestern Canada with a high fluvial sediment input (21.2-25.5 Mtyr-1). Descriptions on sub-environments, stratification, and sediment accumulation within the Skeena Estuary utilize HydroTrend model outputs of riverine sediment and discharge, Natural Resources Canada radiocarbon-dated sediment cores and grain size samples, and acoustic Doppler current profiler and conductivity-temperature-depth measurements from three field campaigns. Research findings delineate a fragmented delta structure with elongated mudflats and select areas of slope instability. Variations from well-mixed water circulation to lateral stratification, govern the slack tide flow transition and sediment transport pathways within seaward and landward passages of the estuary. -
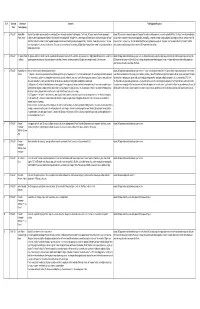
ID # Comment Commenter Comment Final Proponent Response Date Name / Agency
ID # Comment Commenter Comment Final Proponent Response Date Name / Agency 1 2017-02-07 Antonia Mills - People in Digby Island region are greatly concerned about the environmental and human health impacts of the Aurora LNG project because Nexen is proposing to Aurora LNG employed a conservative approach throughout the environmental assessment to ensure the potential effects of the Project were not underestimated. Prince George dredge and alter a large portion of the Delusion Bay habitat for their loading trestle. This project could have impacts to Skeena Salmon similar to those posed by Pacific As outlined in the environmental assessment application, Delusion Bay is expected to remain largely undisturbed and dredge activities are limited to areas off of North West LNG at Flora Bank. Dr. Barb Faggetter assigned a habitat value to the Delusion Bay area equal to that of Flora Bank. Studies being carried out in the area Frederick Point and Casey Cove. The Fish Habitat Offset Plan (currently being developed) will include details on the potential effects to fish and fish habitat have shown significant numbers of juvenile salmon. The project is also very close to the community of Digby Island. A proper Environmental Review would note that the including salmon and the proposed offset to meet the DFO requirement of no net loss. salmon need protection. 2 2017-02-07 Christopher Travis The proposed LNG terminal will hamper the migration of salmon and steelhead up the Skeena River. Any encroachment on Digby Island will have a deleterious affect on Aurora LNG appreciates and acknowledges your concerns. Aurora LNG is undergoing a thorough, independent environmental assessment process, led by the - California spawning salmon and also growth of juvenile salmon and steelhead.