Preterm Birth Or Small for Gestational Age in a Singleton Pregnancy and Risk of Recurrence in a Subsequent Twin Pregnancy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Human Chimera: Legal Problems Arising from Individuals with Multiple Types of Dna
Seton Hall University eRepository @ Seton Hall Law School Student Scholarship Seton Hall Law 5-2-2014 The umH an Chimera: Legal Problems Arising From Individuals with Multiple Types of DNA Robert Russell Granzen Follow this and additional works at: https://scholarship.shu.edu/student_scholarship Recommended Citation Granzen, Robert Russell, "The umH an Chimera: Legal Problems Arising From Individuals with Multiple Types of DNA" (2014). Law School Student Scholarship. 485. https://scholarship.shu.edu/student_scholarship/485 THE HUMAN CHIMERA: LEGAL PROBLEMS ARISING FROM INDIVIDUALS WITH MULTIPLE TYPES OF DNA Robert Granzen INTRODUCTION Science continually changes, and with it our understanding of the human body. While some scientific developments are limited in scope, others have widespread effects. Scientists have just recently begun understanding the range of effects chimerism in humans can have. Chimerism, originally associated with hermaphrodites having both male and female sexual organs, is much more common than originally thought. As chimerism becomes more common, so do individuals with separate and distinct deoxyribonucleic acid (DNA) strands in their bodies. Most individuals are unaware of their chimeric genetic code and most will likely never know. Because of the inherent difficulty of testing for chimerism, many problems are presented to legal system. Part I of this article will begin with the history of human chimeras. The section will then describe the ways in which chimeras are formed. The section will discuss the most common form of chimerism in humans--fetal cell microchimerism (FMC). FMC occurs when cells are transferred from baby to mother or mother to baby via the umbilical cord.1 Studies have shown that mothers may keep cells from their children for years after giving birth.2 Moreover, cells can be exchanged between twins while inside the uterus.3 Secondly, the section will describe the process of embryo fusion, which can cause tetragametic chimerism. -

Association Between First-Trimester Intrauterine Hematoma and Twin Pregnancy Outcomes: a Retrospective Cohort Study
Association Between First-Trimester Intrauterine Hematoma and Twin Pregnancy Outcomes: A Retrospective Cohort Study Wanqing Ji Guangzhou Women and Children's Medical Center Jie Zheng Guangzhou Women and Children's Medical Center Weidong Li Guangzhou Women and Children's Medical Center Fang Guo Guangzhou Women and Children's Medical Center Bo Hou Third Aliated Hospital of Sun Yat-Sen University Ping He ( [email protected] ) Guangzhou Women and Children's Medical Center https://orcid.org/0000-0002-6551-8578 Research article Keywords: intrauterine hematoma, twin gestation, rst trimester, miscarriage, vanishing twin syndrome Posted Date: September 21st, 2020 DOI: https://doi.org/10.21203/rs.3.rs-75567/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Version of Record: A version of this preprint was published on January 11th, 2021. See the published version at https://doi.org/10.1186/s12884-020-03528-0. 1 Title page 2 Association between first-trimester intrauterine hematoma and twin pregnancy 3 outcomes: A retrospective cohort study 4 Wanqing Ji1,Ϯ , jie Zheng1,Ϯ, Weidong Li 2,Ϯ, Fang Guo1, , Ping He1,* Bo Hou3,* 5 1Department of Obstetrics, Guangzhou Women and Children’s Medical Center, Guangzhou Medical 6 University, Guangzhou, 510623,China. 7 2Department of Woman and Child Health Information Guangzhou Women and Children’s Medical 8 Center, Guangzhou Medical University, Guangzhou, 510623,China. 9 3Departments of Neurosurgery, The Third Affiliated Hospital, Sun Yat-sen University, Guangzhou, 10 Guangdong province, 510630, China.E-mail: [email protected] 11 *Correspondence address. Ping He, Guangzhou Women and Children’s Medical Center, Guangzhou 12 Medical University, Guangzhou, Guangdong province, 510623, China.Fax number: 86-20-8717 6790 13 E-mail: [email protected] 14 Bo Hou , Departments of Neurosurgery, The Third Affiliated Hospital, Sun Yat-sen University, 15 Guangzhou, Guangdong province, 510630, China. -

CDC Having Healthy Babies One at a Time
HAVING HEALTHY BABIES ONE AT A TIME Why are we worried about twin pregnancies? We know that you are ready to start or add to your family. You may be concerned about your chances of having a baby using in vitro fertilization (IVF) or how much cycles of IVF cost. These concerns are common and may lead you to think about transferring more than one embryo during your IVF procedure. However, transferring more than one embryo increases your chances of having twins or more. Twin pregnancy is risky for baby and mother, whether or not IVF is used. Some of these risks include: Almost 3 out of 5 Twin babies are more likely to be stillborn, twin babies are born preterm, or at less than experience neonatal death, have birth defects of 37 weeks of pregnancy. Twin babies are nearly the brain, heart, face, limbs, muscles, or digestive 6 times as likely to be born preterm as system, and have autism than single babies. single babies. Almost 1 out of 10 About 1 out of 4 women carrying twins gets pregnancy-related twin babies are admitted to the neonatal high blood pressure. Women carrying twins intensive care unit (NICU). Twin babies are are twice as likely to get pregnancy-related high more than 5 times as likely to be admitted to the blood pressure as women carrying single babies. NICU as single babies. Almost 1 out of 20 About 7 out of 1,000 women carrying twins gets gestational diabetes. twin babies have cerebral palsy. Twin babies are Women carrying twins are 1.5 times as likely to get more than 4 times as likely to have cerebral palsy gestational diabetes as women carrying as single babies. -

Association Between Chorionicity and Preterm Birth in Twin Pregnancies: a Systematic Review Involving 29 864 Twin Pregnancies
DOI: 10.1111/1471-0528.16479 Systematic Review www.bjog.org Association between chorionicity and preterm birth in twin pregnancies: a systematic review involving 29 864 twin pregnancies S Marleen,a,b C Dias,b R Nandasena,b R MacGregor,c J Allotey,d J Aquilina,c A Khalil,e,f S Thangaratinamg a Barts Research Centre for Women’s Health (BARC), Barts and the London School of Medicine and Dentistry, Queen Mary University of London, London, UK b Sri Jayewardenepura Postgraduate Teaching Hospital, Nugegoda, Sri Lanka c Royal London Hospital, Barts Health NHS Trust, London, UK d Institute of Applied Health Research, University of Birmingham, Birmingham, UK e St George’s University Hospitals NHS Foundation Trust, London, UK f Molecular and Clinical Sciences Research Institute, St George’s Medical School, University of London, London, UK g World Health Organization (WHO) Collaborating Centre for Global Women’s Health, Institute of Metabolism and Systems Research, University of Birmingham, Birmingham, UK Correspondence: S Marleen, Barts Research Centre for Women’s Health (BARC), Barts and the London School of Medicine and Dentistry, Queen Mary University of London, Mile End Road, London E1 4NS, UK. Email: [email protected] Accepted 7 August 2020. Published Online 7 October 2020. Background The perinatal mortality and morbidity among twins I2 = 46%, OR 1.55, 95% CI 1.27–1.89 I2 = 68%, OR 1.47, 95% CI vary by chorionicity. Although it is considered that 1.27–1.69, I2 = 60%, OR 1.66, 95% CI 1.43–1.93, I2 = 65%, monochorionicity is associated with an increased risk of preterm respectively). -

Pre-Eclampsia/Eclampsia in Twin Pregnancies A
J Med Genet: first published as 10.1136/jmg.13.3.208 on 1 June 1976. Downloaded from Journal of Medical Genetics (1976). 13, 208-211. Pre-eclampsia/eclampsia in twin pregnancies A. McFARLANE and J. S. SCOTT From the Department of Obstetrics and Gynaecology (Leeds Maternity Hospital), University of Leeds, 17 Springfield Mount, Leeds LS2 9NG Summary. A study of 1045 twin gestations with regard to known or likely zygosity and the incidence of pre-eclampsia/eclampsia failed to reveal differences between known dizygous twins and like-sex 'presumed' and 'estimated' monozygous twins except in the 'estimated' data for multigravidae. There was a threefold in- crease in the incidence for twins as opposed to singleton pregnancies. These results are discussed in relation to increased conceptus-mother antigenic differences. It is suggested that the risk of gestosis in twin pregnancy involves more than a summa- tion ofthat operating in two singleton pregnancies. It has been suggested that genetic incompatibility Pregnancies were classified according to definitions given between mother and fetus may be a factor in the below. aetiology of pre-eclampsia (Penrose, 1946; Kalmus, 1946; Platt, Stewart, and Emery, 1958). Epidemio- A. Hypertension status logical evidence has been provided by Stevenson et (1) Mildpre-eclampsia-a basal blood pressure of 120/ 80 mm Hg or less recorded before 24 weeks' gestation, al (1971) in a study of consanguineous marriages in followed by a rise to 140/90 mm Hg or more on at least the Middle East. They also recorded twin data two occasions recorded in the antenatal ward, clinic which pointed to a higher incidence of toxaemia in readings being discounted, together with oedema and unlike-sex as opposed to like-sex twin pregnancies. -

Twin to Twin Transfusion Syndrome (TTTS)
CENTER FOR FETAL THERAPY The Johns Hopkins Hospital 600 North Wolfe Street, Nelson Building, Suite 228 Baltimore, MD 21287 Phone: 410 502 6561 Email: [email protected] Twin-twin transfusion syndrome Important things to know What is twin-twin transfusion or twin anemia polycythemia syndrome? Twin twin transfusion syndrome (TTTS) complicates up to 15% of identical twins. It is due to unequal sharing of blood volume across blood vessel connections in a monochorionic placenta. Twin anemia polycythemia sequence (TAPS) complicates 4% of identical and is due to unequal sharing of blood cells. How do TTTS and TAPS present or harm the babies? In TTTS the donor twin has low blood volume (essentially dehydration) while the recipient has a high blood volume (overhydrated). The dehydrated donor drinks up the amniotic fluid and produces less and less urine. Eventually this leads to a progressive emptying of its amniotic sac until the donor becomes “stuck” wrapped in his own membrane. The recipient twin does the opposite, urinating so much that a massive amount of amniotic fluid is generated distending the amniotic sac and uterus. Eventually TTTS can lead to heart failure or even death in either twin. In other circumstances the massive increase in amniotic fluid can distend the uterus so much that preterm labor and birth of both twins may be triggered. In TAPS donor has thin blood and is anemic, which results in higher than normal blood flow speed. The recipient has thick, slow flowing blood, with a high red cell count (polycythemia). The anemic donor twin is eventually at risk for heart failure and death. -

Polyploidy, Infraspecific Cytotype Variation, and Speciation in Goldenrods: the Cytogeography of Solidago Subsect
TAXON 61 (1) • February 2012: 197–210 Peirson & al. • Cytogeography of Solidago subsect. Humiles Polyploidy, infraspecific cytotype variation, and speciation in Goldenrods: The cytogeography of Solidago subsect. Humiles (Asteraceae) in North America Jess A. Peirson,1,2 Anton A. Reznicek2 & John C. Semple3 1 Department of Ecology and Evolutionary Biology, The University of Michigan, Ann Arbor, Michigan 48109-1048, U.S.A. 2 The University of Michigan Herbarium, 3600 Varsity Drive, Ann Arbor, Michigan 48108-2228, U.S.A. 3 Department of Biology, University of Waterloo, Waterloo, Ontario N2L 3G1, Canada Author for correspondence: Jess A. Peirson, [email protected] Abstract Polyploidy is an important evolutionary mechanism in plants, and in some genera (e.g., Solidago in Asteraceae) it is particularly widespread and is hypothesized to have played a major role in diversification. Goldenrods are notorious for their ploidy variation, with roughly 14% and 32% of recognized North American species being polyploid or including multiple cytotypes, respectively. We used traditional chromosome counts and flow cytometry to examine cytogeographic patterns, biogeographic and evolutionary hypotheses, and species boundaries in S. subsect. Humiles. Chromosome numbers and DNA ploidy determinations are reported for 337 individuals, including 148 new reports. Cytotypes show significant geographic struc- turing. Solidago simplex and S. spathulata were uniformly diploid (2n = 18) in western North America, while cytogeographic patterns in eastern North America were regionally complex and included 2n, 4n, and 6n cytotypes. Cytotypes within S. simplex were ecogeographically segregated and mixed-ploidy populations were rare. Data from this study and additional biosystematic data indicate that cytotypes in S. simplex fulfill the requirements of multiple species concepts and should best be treated as distinct species. -

“But I Want Twins” …But What Are the Risks?
“But I want Twins” …but what are the risks? A large proportion of patients (>40%) undergoing IVF wish for a multiple pregnancy, not just as an acceptable outcome, but as the desired outcome for their pregnancy. However, there are considerable, well known risks to multiple pregnancy - even twin pregnancy - as a recent letter from one of our patients to her physician here at Shady Grove Fertility illustrates: “Sorry we haven’t been in touch sooner, but the girls really threw us a curveball ...The girls were born at just over 25 weeks, weighing in at 900 grams and at 860 grams. Being so premature, there have been a lot of challenges, and one unfortunately developed necrotizing enterocolitis at 32 weeks which required surgery back in May. The other had a “scare” but did not wind up being treated…They’re absolutely lovely and beautiful and really interacting, smiling and cooing, which is a delight after such a difficult journey these past months. They are moving up from the very lowest percentiles on the growth charts for weight and length. We’ve been through more than I can tell you…Having spent the lion’s share of the past 7 months daily in hospital and seeing such an awfully different beginning than I would have chosen for the girls (or us)…I believe it would be beneficial to educate those considering multiple transfers about the risks of preterm labor/premature birth/prematurity (using all of its names and encouraging research)…Thanks so much to all of you at Shady Grove for the help and dedication and please thank our donor for her amazing gift that allowed us to realize our dream.” With its well know risks to mothers and infants, the decision between seeking a twin pregnancy vs. -

Twin Studies in Medical Genetics
7. TWIN STUDIES IN MEDICAL AND CLINICAL GENETICS TWIN STUDIES IN MEDICAL GENETICS P. PROPPING, F. VOGEL Institute of Anthropology and Human Genetics, University of Heidelberg, German Federal Republic It is the aim of twin studies to obtain results which are not only valid for twins, but apply to the whole population. Therefore the following questions have to be answered first: do twins differ from non- twins in the trait under study ? Do different nongenetic factors act upon MZ and DZ twins which alter the probability of manifestation of a trait, even before birth ? There are important differences in embryo- genesis and placental blood flow in mono- and dichorionic twins; this can influence the normal fetal development. Therefore the value of twin studies alone in analysing the genetic component in the etiology of congenital malformations is rather ambiguous. Twin studies beyond the newborn period can be classified into four approaches: (1) Case reports; (2) Accumulated case reports; (3) Limited representative sample; (4) Unlimited representative sample. The most frequent systematic method in medical genetics is the establishment of all twins in a defined population of probands {3). Another successful application in the last few years has been in pharmacogenetics. Although no simple mode of inheritance could be dis covered, it was possible to estimate the genetic component within the interindividual variability of the metabolism of certain drugs {nortriptyline, antipyrine, phenylbutazone, ethanol). Now, additional non- twin research is needed to work out single factors within the observed polygenic systems. We will limit our considerations on " Twins in Medical Genetics " to the following aspects: 1. -

Twins Reared Together and Apart: the Science Behind the Fascination1
Twins Reared Together and Apart: The Science Behind the Fascination1 NANCY L. SEGAL Professor of Psychology and Director Twin Studies Center California State University, Fullerton he reasons behind the widespread interest in twins are fascinating to consider. There is no question that people are intrigued by T twins—wherever I go I am asked about my work by both profes- sionals and non-professionals. I often ask them, “Why do you care about this topic?” Most of them reply, “Well, it is just so interesting,” but no one can provide a more substantive answer. I have thought a great deal about this question. I believe that twins are fascinating because we expect and anticipate individual differences in behavior and appearance. There- fore, when we encounter two people who look so much alike and act so much alike, it becomes intriguing because it challenges our beliefs in the way the world works. For some people, this may be disturbing, but for many, it is quite compelling. There are also many misunderstandings surrounding the origins of twinning and twin development. I address some of them in this paper and many others in my new book Twin Mythconceptions (Segal, 2017). Two Twin Types The study of twins rests on the availability of two types of twins. Identical, or monozygotic (MZ), twins share all of their genes in common, having split from a single fertilized egg between the first and fourteenth post-conceptional day (Segal, 2000). Fraternal, or dizygotic (DZ), twins result from the fertilization of two separate eggs by two separate sperm and share 50% of their genes, on average, by descent, just like full siblings. -
Managing Risk—To Mother and Fetuses—In a Twin Gestation
Victoria Belogolovkin, MD, and Joanne Stone, MD Dr. Belogolovkin is a fellow and Dr. Stone is associate professor in the Department of Maternal–Fetal Medicine, Mount Sinai School of Medicine, New York, NY The authors report no fi nancial relationships relevant to this article. Dichorionic twins at 11+ weeks' gestation. Nuchal translucency is increased in the twin at right, LaRocco ® signaling that it may be at risk Rich Dowden Health Media © of aneuploidy. Copyright ManagingFor personal risk—to use only mother and fetuses—in a twin gestation IN THIS ARTICLE ❙ Monochorionic Begin by determining chorionicity. Discuss the risks twins are at risk of of a multiple gestation, prepare parents for premature serious problems delivery, and monitor fetal growth as indicated. Page 67 ❙ Does prenatal test- ultiple gestations are far more The diffi culties that twins encounter are ing raise the threat common today than they once often associated with preterm birth and Mwere—up 70% since 1980.1 To- occur most often in identical twins de- of miscarriage with day, every 1,000 live births include 32.3 veloping within a single gestational sac. multiples? sets of twins, a 2% increase in the rate Those diffi culties include malformation, Page 77 of twin births since 2004. Why this phe- chromosomal abnormalities, learning nomenon is occurring is not entirely un- disability, behavioral problems, chronic ❙ Fetal reduction: derstood but, certainly, the trend toward lung disease, neuromuscular develop- An option for some older maternal age and the emergence of mental delay, cerebral palsy, and still- parents assisted reproduction are both part of the birth. -
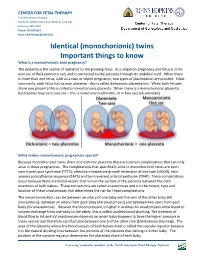
Monochorionic Twins Share One Common Placenta There Are Certain Complications That Can Only Arise in These Pregnancies
CENTER FOR FETAL THERAPY The Johns Hopkins Hospital 600 North Wolfe Street, Nelson Building, Suite 228 Baltimore, MD 21287 Phone: 410 502 6561 Email: [email protected] Identical (monochorionic) twins Important things to know What is a monochorionic twin pregnancy? The placenta is the source of nutrients to the growing fetus. In a singleton pregnancy one fetus is in his own sac of fluid (amniotic sac) and is connected to the placenta through its umbilical cord. When there is more than one fetus, such as a twin or triplet pregnancy, two types of placentation are possible. Most commonly, each fetus has its own placenta - this is called dichorionic placentation. When both fetuses share one placenta this is called a monochorionic placenta. When there is a monochorionic placenta both babies may be in one sac – this is called mono-amniotic, or in two sacs (di-amniotic). What makes monochorionic pregnancies special? Because monochorionic twins share one common placenta there are certain complications that can only arise in these pregnancies. The complications that specifically arise in monochorionic twins are twin- twin transfusion syndrome (TTTS), selective intrauterine growth restriction of one twin (sIUGR), twin anemia polycythemia sequence (TAPS) and twin reversed arterial perfusion (TRAP). These complications occur because there are blood vessels that run on the surface of the placenta between the cord insertions of both babies. These connections are called anastomoses and it is the nature, type and location of these anastomoses that determines the risk for these complications. The vessel connections can be between an artery of one baby and the vein of the other baby (AV anastomosis), between an artery from each baby (AA anastomosis) and between two veins from each baby (VV anastomosis).