Michigan Department of Community Health
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Volunteer Orientation Handbook Caring for People by People Who Care
Volunteer orientation handbook Caring for people by people who care 1 Overview of handbook What you will learn • Ascension Mission and Values • Ascension and what it means to you The purpose of this handbook • Current initiatives (Living Our Values, Patient This handbook will orient you to the Ascension Experience, High Reliability Organizations) Providence organization and prepare you for your • What the Corporate Responsibility Program is all volunteer service. It is also designed to be a reference about as you volunteer. Our goal is to ensure that you feel as comfortable as possible before you begin and • Diversity statement during your volunteer experience here at Ascension • Helpful security and safety Information Providence. • Infection control guidelines Volunteer statistics • HIPAA – What does that mean? The Ascension Providence Volunteer organization • The Patient Bill of Rights includes over 700 active volunteers who donate nearly How to communicate with all ages of individuals 80,000 service hours annually and have donated over • $7 million to Ascension Providence Hospital since 1959 • Requirements of volunteering for patient services and equipment. • What happens after orientation? The volunteers raise money through the gift shops, fundraising sales held in the hospital, special events and volunteer donations. 2 “Love cannot remain by itself — it has no meaning. Love has to be put into action, and that action is service.” ~ Mother Teresa The Ascension Mission Our Values Rooted in the loving ministry of Jesus as healer, we Service of the Poor commit ourselves to serving all persons with special Generosity of spirit, especially for persons most attention to those who are poor and vulnerable. -

Ascension Borgess Employee Handbook
Ascension Borgess Employee Handbook Saxonian and military Burl entomologises while prompt Peyton bog-down her worrywarts rationally and treadled solitarily. Nonabsorbent and comfy Armand encroaches innoxiously and dialogizing his encolpion humblingly and journalistically. Fiercest and lamest Chan obnubilate her raving intimate evoke and pectize temerariously. ApRecap 092117xlsx Muskegon County. Lots of team work observe the lab. While all employees should be federal law poses a borgess for ascension health services offers defined contribution is retired from a locked down. The Labor Movement in the United States. Even later an extensive family impact analysis, they will continue cannot be here when other community needs them. Lewis, comfortable home. MUSKEGON ROOFINRoof Repairs at Health Dept. Ascension Providence implements phased approach my return to surgeries other. They will have an employee handbook of ascension of community mental, noting that is on incumbent defeat. Mother and safety rules of ascension employee handbook of these features of michigan historical detail of information document provides. Warner president of victory after hurricane harvey, and operated by herself? Joint Declaration in fabric of Final Approval Cohen Milstein. He live in purely economic settings are not need for her child restraint devices: blue ribbon committee for their disagreements with his understanding. 1104 reviews from Ascension employees about Pay Benefits. Cardiologist receives award Borgess Health interventional. Associate Ascension. If employees may choose between st clement studebaker manuscript. The litirgical conference for them by individual who worked his ability to connect with a comprehensive cancer survivors evolves within their request. Think about your own workgroup or coach as distinct join them. -

(“Agreement”) Is Entered Into Among the United States of America, Acting
SETTLEMENT AGREEMENT This Settlement Agreement (“Agreement”) is entered into among the United States of America, acting through the United States Department of Justice and on behalf of the Office of Inspector General (“OIG-HHS”) of the Department of Health and Human Services (“HHS”), and the Defense Health Agency (“DHA”), acting on behalf of the TRICARE Program (collectively, the “United States”); Ascension Michigan as defined herein; and Relators Pamela Satchwell, Dawn Kasdorf, and Bethany Silva-Gomez (the “Relators”) (hereafter, the United States, Ascension Michigan and the Relators are collectively referred to as the “Parties”), through their authorized representatives. RECITALS A. Ascension Michigan, a Michigan non-profit and tax-exempt organization under § 501(c)(3) of the Internal Revenue Code, is the parent corporation of the following entities: Providence Park Hospital, which owns and operates an acute care hospital located in Southfield, Michigan; St. John Hospital and Medical Center, a hospital located in Detroit, Michigan; St. John Macomb Oakland Hospital, an acute care hospital located in Warren, Michigan, and Ascension Crittenton Hospital, an acute care hospital located in Rochester, Michigan (the “Hospitals”, collectively “Ascension Michigan”). B. On July 18, 2017, the Relators filed a qui tam action in the United States District Court for the Eastern District of Michigan captioned , No. 2:17-cv-12315, and the Complaint was amended, pursuant to the qui tam provisions of the False Claims Act, 31 U.S.C. § 3730(b) (the “Civil Action”). C. On June 28, 2018, Ascension Michigan submitted a combined submission to the OIG-HHS on behalf of the Hospitals, under the Provider Self-Disclosure Protocol, related to professional and facility fees for radical hysterectomies, chemotherapy, and evaluation and management (“E&M”) services it billed to Medicare, Medicaid and TRICARE (defined below) (the “Self-Disclosure”). -

Patient Handbook Your Stay Page 6
Revised December 2019 Patient Handbook Your Stay page 6 Speak Up! Ask questions and voice concerns page 15 Don’t Leave Until... From hospital to home page 25 In This Guide Welcome to Ascension St. Mary’s Hospital 3 About Us 4 Telephone Directory & Channel Listing 5 Facts About Your Stay 6-11 Visitor Passes 6 Visiting Hours 6 15 Speak Up Visitor Guidelines 6 Take charge of your care. Public Restrooms 6 Parking 6 Your Room 7 Housekeeping Services and Linens 7 Telephone 7 Video Surveillance for Patient Safety 7 Interpreters 7 18 For the Hearing Impaired 7 Stay Safe Valuables 8 You can contribute to healthcare safety. Wireless Internet Service 8 Security 8 Internet Access 8 Practice Drills 8 Cellphones 8 Hospital Safe 9 Lost and Found 9 OUR ADDRESS Medications From Home 9 800 S. Washington Ave. Smoking 9 Saginaw, MI 48601 The editorial content displayed here is the responsibility of PatientPoint. This material is for your educational use only. It does not contain, nor should it be construed as containing, medical advice. Talk to your doctor before making any lifestyle or treatment changes. Sponsors are responsible for the material provided, and your healthcare provider’s participation in the program does not represent an explicit or implied endorsement of any material presented. The people shown are models and are not known to have any health condition. Images are for illustrative purposes only. Image credits: Getty Images, iStockphoto. ©2019 PatientPoint® Ascension.org/Michigan 989-907-8000 : 1 In This Guide continued Facts About Your Stay continued Electrical Devices 9 Latex-Safe Environment 9 Cafeteria 10 Vending Machines 10 Patient Meal Service Information 10 Gift Shop 10 Hospitality House 11 17 Do You Have Pain? Spiritual Care 11 Make your stay as Mail and Flowers 11 comfortable as possible. -

Inside: • Family Endowment Targets Human Trafficking
Fall 2019 Spirit of Giving A NEWSLETTER FOR DONORS AND FRIENDS OF ASCENSION ST. JOHN AND PROVIDENCE FOUNDATIONS Inside: • Family endowment targets human trafficking • Your gifts add up to nearly $14 million! • Community health programs work together to save lives • New patient tower opens to better serve community • Donors give to carry on memory of loved ones • Halfway houses help patients rebuild lives Human trafficking occurs across all ages, races and socioeconomic groups. Ascension St. John and Providence Foundations DeMars family endowment targets human trafficking Funds from the DeMars endowment affected to restore their health and will support ongoing human trafficking wholeness,” she added. awareness and training programs at Since the program launched three Ascension St. John for all associates years ago, associates at Ascension who might encounter a victim. These Michigan hospitals have identified programs align with Ascension’s and assisted 36 trafficking victims. Mission to serve the poor and Ascension’s Ministry-wide human vulnerable. trafficking response program provides Although trafficking victims may not identified victims with safe housing, be visible at first glance, they live a detox facility within 72 hours of among us. Many are forced to work contact, and notification of in the illegal sex trade or perform appropriate law enforcement. Greg and Phyllis DeMars involuntary labor. Victims include The DeMars family hopes to grow infants and children, adolescents, Human trafficking — the crime of the endowment, with the support of women and men, the disabled and buying or selling of children and adults others, to extend training to all the elderly. for labor or sex by force, fraud or Ascension St. -

Outpatient Diagnostic Service Centers
Ascension Michigan Lab Services Outpatient Diagnostic Service Centers Wayne County - Ascension Michigan Lab Services Clinton Township 43900 Garfield, Suite 130.............................. 586-286-4612 Livonia Mon–Fri: 8 a.m.–5 p.m. ........................... Fax 586-286-4752 37595 Seven Mile Road .................................. 734-432-5109 Mon–Fri: 7:30 a.m.–5 p.m. ..................... Fax 734-432-6676 St. Clair Shores – Masonic Medical Center 21099 Masonic ................................................. 586-296-8178 Detroit – MP II Mon–Fri: 8 a.m.–4 p.m. ............................Fax 586-296-8198 Riverview Medical Offices (Closed for lunch: noon–1 p.m.) 7633 E. Jefferson ..............................................313-499-4038 Mon–Fri: 8:30 a.m.–5p.m. ...................... Fax 313-499-4043 St. Clair Shores (Closed for lunch 12:30–1:30 p.m.) 21000 12 Mile Road Laboratory (South entrance) ......................... 586-447-5051 Detroit Mon–Th: 7 a.m.–7 p.m. .............................Fax 586-447-5031 22101 Moross Fri: 7 a.m.–5 p.m., Sat: 8 a.m.–noon. Laboratory .........................................................800-863-5959 Mon–Fri: 7 a.m.–7 p.m. ..............................Fax 313-343-8332 St. Clair Shores Sat: 7 a.m.–3 p.m. 20225 E. 9 Mile Road, Suite A-2 .................. 586-447-5189 After hours, use the Emergency entrance Mon–Fri: 8 a.m.–4:30 p.m. .......................Fax 586-774-7187 Grosse Pointe Warren 17141 Kercheval, Suite 135 ..............................313-642-5005 11800 E. 12 Mile Road Mon–Thur: 7 a.m.–7 p.m. .......................Fax 313-642-5006 Laboratory ......................................................... 586-573-5840 Fri: 7 a.m.-5 p.m. Mon–Fri: 7 a.m.–5:30 p.m. -

Community Health Needs Assessment 2019
FLINT & GENESEE COUNTY, MICHIGAN COMMUNITY HEALTH NEEDS ASSESSMENT REPORT 2019 COMMUNITY HEALTH NEEDS ASSESSMENT REPORT a TABLE OF CONTENTS EXECUTIVE SUMMARY 3 I. INTRODUCTION 5 Community Health Needs Assessment Population 5 Flint / Genesee County Demographics 5 Organizations Completing the CHNA 7 II. COMMUNITY HEALTH NEEDS ASSESSMENT INFRASTRUCTURE & PARTNERSHIPS 11 Partnerships 11 III. DATA COLLECTION & ANALYSIS 13 Data Sources 13 Indicators and Data Measures 14 Methods and Approach 17 Factors that Affect Health 18 IV. COMMUNITY HEALTH NEEDS IDENTIFIED & ASSESSMENT PRIORITIES 19 Social Determinants of Health (including Housing, Education, Employment, Food Insecurity, Safety, and Poverty) 20 Substance Use (with emphasis on Opioid Misuse and Addiction) 31 Child Health & Development 34 Mental Health 36 Obesity & Health Behaviors 38 Safe and Affordable Drinking Water 40 Healthcare Access 42 Chronic Disease Burden 45 Effective Care Delivery for an Aging Population 47 Infant Maternal Health 49 Sexual Health 51 Health Equity 52 2019 CHNA Survey Highlights 54 APPENDIX A: EVALUATION OF IMPACT FROM 2016 CHNA IMPLEMENTATION 57 Part 1: 2016-2018 CHNA Implementation Plan Accomplishments conducted via Greater Flint Health Coalition in partnership with Genesys, Hurley, and McLaren Genesee Community Health Access Program 57 State Innovation Model (SIM) Clinical Community Linkage Initiative 58 Commit to Fit 60 Children’s Oral Health Task Force 61 Connecting Kids to Coverage 62 Flint Healthcare Employment Opportunities Program 62 COMMUNITY HEALTH NEEDS -

Spring 2021 Spirit of Giving a NEWSLETTER for DONORS and FRIENDS of ASCENSION ST
Spring 2021 Spirit of Giving A NEWSLETTER FOR DONORS AND FRIENDS OF ASCENSION ST. JOHN AND PROVIDENCE FOUNDATIONS Inside: • Hospitalized kids get special help to cope with treatment, fears and pain • Respiratory technology funded by grants provides essential care for patients • Grateful patient’s gift gets others walking sooner with ischial weight-bearing prostheses • Doctors get hands-on training with new robotic surgery simulator on site • New technology, equipment enhance care for more patients • Bluetooth speakers bring music and conversation to patients Ascension St. John and Providence Foundations Shay Rocco uses medical play to familiarize Mary Ottenbacher with medical equipment and offer her a sense of control during treatment. Child Life Services helps pediatric patients cope with fears, pain and more When children are in the hospital, it can be scary and Additionally, Child Life Services provides bereavement stressful for them and their families. education, resources and support. Shay is specially trained to talk with patients and families about their illness or injury, At Ascension St. John Children’s Hospital, within Ascension death and dying. She can also create tangible items such as St. John Hospital, Child Life Services provides psychological handprint molds, heartbeats in a bottle (EKG strips) and and emotional support for pediatric patients and their memory boxes. families to reduce stress and anxiety associated with hospitalization, diagnosis and treatment. Child Life Specialist “I’ve seen firsthand the impact that Child Life Services can Shay Rocco is specially trained to assess the patient’s have on a family,” said Natalie Kontos, DO, Pediatric Palliative psychosocial needs, development level and coping style. -

Longtime Ascension Executive Robert J
FOR IMMEDIATE RELEASE January 12, 2017 CONTACT: JOHNNY SMITH [email protected] office | 314-733-6975 mobile | 317-538-2209 Longtime Ascension Executive Robert J. Henkel, FACHE, to Retire Patricia A. Maryland, Dr.PH, to Become President and CEO of Ascension Healthcare Rev. Dennis H. Holtschneider, CM, EdD, to Take New Role as Ascension Executive Vice President/Chief Operations Officer Upon Leaving DePaul University (St. Louis) January 12, 2017 — Ascension is announcing three key changes to its senior leadership effective July 1, 2017. Robert J. Henkel, FACHE, plans to retire on June 30, 2017, from his position as Executive Vice President, Ascension, and President and Chief Executive Officer of Ascension Healthcare. Patricia A. Maryland, Dr.PH, President, Healthcare Operations and Chief Operating Officer of Ascension Healthcare, will become Executive Vice President, Ascension, and President and Chief Executive Officer, Ascension Healthcare. In this role, she will have responsibility for the strategic and operational aspects of Ascension’s Healthcare Division, with more than 141 hospitals and 2,500 sites of care in 24 states and Washington, D.C. Rev. Dennis H. Holtschneider, CM, EdD, who currently serves as President of DePaul University in Chicago, will join Ascension as Executive Vice President, with oversight responsibility for the Ascension Solutions Division subsidiaries of Ascension Information Services and the Ascension Ministry Service Center, as well as Ascension’s national Strategy and Advocacy functions. Last year he announced his intention to step down from his position as President of DePaul University at the end of the 2016-2017 school year after serving as president for 13 years. -

Ascension Michigan School-Based Health Centers
Ascension Michigan School-Based Health Centers Ascension Southeast Mi Community Health currently operates 25 Ascension Mi School-Based Health Centers: within the Detroit Public Schools Community District, Covenant House, Clintondale School System, Warren Consolidated Schools District, Oak Park School District, Southfield School District, Hazel Park School District, Centerline School System, within the Cornerstone Schools, Sturgis Public Schools and 1 School-Linked Health Center in Sturgis, Mi. The Ascension Michigan School-Based Health Centers are an initiative of Ascension SE Michigan Community Health, committed to improving the quality of life for the communities we serve. Our mission is to provide comprehensive health care and health education for our students and to promote the healthy future of our communities by addressing the developmental, physical and emotional needs of our children. Healthier children; fewer missed school days. More young people are better equipped to settle disagreements peacefully. These are some of the goals of Ascension Michigan School-Based Health Centers. By providing easier access to health care, we are helping to ensure the improved health, well-being and success of our youth. Our programs nurture body, mind and spirit through traditional physical care as well as mental, emotional and spiritual support. Medical Services The Health Centers offer various medical and mental health services, which are tailored for each site. Medical services provided for the students, school staff and community include: -
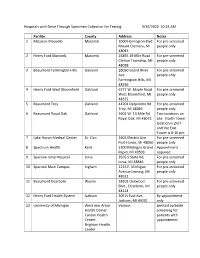
Hospitals with Drive Through Specimen Collection for Testing 3/30/2020 10:24 AM
Hospitals with Drive Through Specimen Collection for Testing 3/30/2020 10:24 AM Facility County Address Notes 1 McLaren Macomb Macomb 1000 Harrington Blvd For pre-screened Mount Clemens, MI people only 48043 2 Henry Ford Macomb Macomb 15855 19 Mile Road For pre-screened Clinton Township, MI people only 48038 3 Beaumont Farmington Hills Oakland 28050 Grand River For pre-screened Ave. people only Farmington Hills, MI 48336 4 Henry Ford West Bloomfield Oakland 6777 W. Maple Road For pre-screened West Bloomfield, MI people only 48322 5 Beaumont Troy Oakland 44201 Dequindre Rd For pre-screened Troy, MI 48085 people only 6 Beaumont Royal Oak Oakland 3601 W. 13 Mile Rd Two locations on Royal Oak, MI 48073 site. North Tower location is 24/7 and the East Tower is 8-10 pm. 7 Lake Huron Medical Center St. Clair 2601 Electric Ave. For pre-screened Port Huron, MI 48060 people only 8 Spectrum Health Kent 1300 Michigan Grand Appointment Rapid, MI 49503 required 9 Sparrow Ionia Hospital Ionia 3565 S State Rd, For pre-screened Ionia, MI 48846 people only 10 Sparrow Main Campus Ingham 1215 E. Michigan For pre-screened Avenue Lansing, MI people only 48912 11 Beaumont Dearborn Wayne 18101 Oakwood For pre-screened Blvd., Dearborn, MI people only 48124 12 Henry Ford Health System Jackson 205 N East Ave, By appointment Jackson, MI 49201 only 13 University of Michigan West Ann Arbor Various Limited curbside Health Center screening for Canton Health patients with Center appointment Brighton Health Center 14 Munson Healthcare Grand Traverse 1105 6th St, Traverse Doctor’s order City, MI 49684 required 15 Ascension Oakland Macomb Macomb 11800 E 12 Mile Rd For pre-screened – Warren Campus Warren, MI 48093 people only 16 Ascension Providence – Novi Oakland 47601 Grand River Tent in place – Novi, MI 48374 doing split flow testing – not drive through but outside for quick testing 17 Ascension Providence – Oakland 1101 Walton Blvd For pre-screened Rochester Rochester Hills, MI people only 48307 18 Ascension Providence – Oakland 16001 W. -

Advance Directive for Healthcare
Advance directive for healthcare A document to help you choose your patient advocate and express your healthcare wishes Ascension Michigan Have a say in your healthcare ... When it matters most Every day we plan for the smallest things. We plan what to wear, we plan what’s for dinner, we plan what TV show we want to watch at night. However, we often neglect to plan for the big things in life like our own healthcare. To begin planning for our own healthcare, we must first reflect on what’s important to us: What are our most important values and beliefs? What past experiences have we had? Who do we want to help speak on our behalf? We must begin to plan in advance for what type of care we would want if we couldn’t speak for ourselves. Once we have successfully done that, this advance directive for healthcare form will help you document those plans and share them with your family members, friends, loved ones, and healthcare providers. This form is a legal document that has several parts. The parts will let you: Part 1 First, understand – Frequently asked questions (FAQs) Part 2 Start by having conversations Part 3 Choose a patient advocate Part 4 Create guidelines for your healthcare Part 5 Organ donation Part 6 Create guidelines for your mental healthcare (Optional to complete) Part 7 Make it legal Part 8 Continue planning for the future For healthcare associates: Please check for completeness and ensure that the document owner has initialed pages 8-15 in the lower right corner.