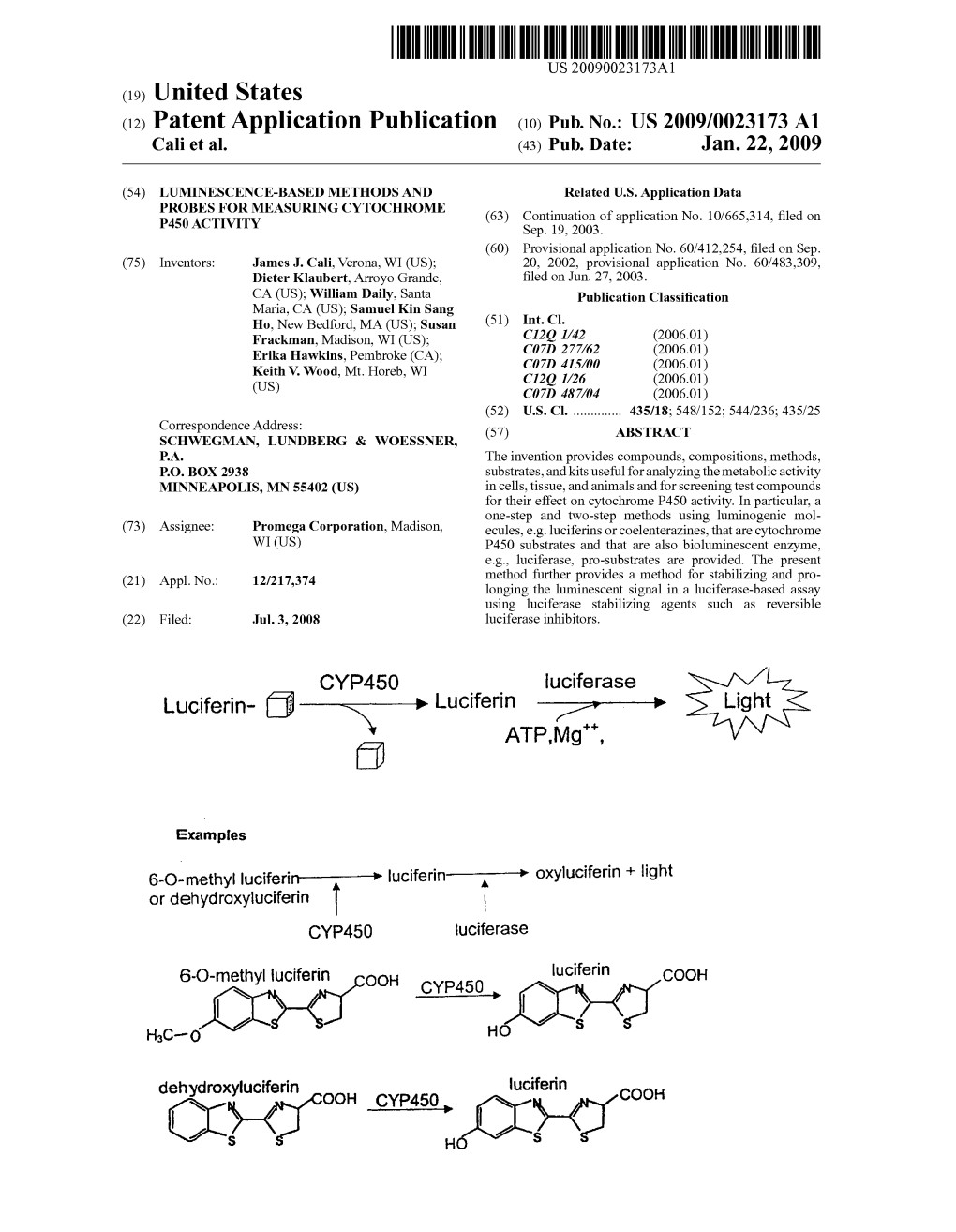
Cyp450 Oc Hc- O Oc)- Ho S
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Saccharomyces Rrm3p, a 5 to 3 DNA Helicase That Promotes Replication
Downloaded from genesdev.cshlp.org on September 24, 2021 - Published by Cold Spring Harbor Laboratory Press Saccharomyces Rrm3p, a 5 to 3 DNA helicase that promotes replication fork progression through telomeric and subtelomeric DNA Andreas S. Ivessa,1 Jin-Qiu Zhou,1,2 Vince P. Schulz, Ellen K. Monson, and Virginia A. Zakian3 Department of Molecular Biology, Princeton University, Princeton, New Jersey 08544-1014, USA In wild-type Saccharomyces cerevisiae, replication forks slowed during their passage through telomeric C1–3A/TG1–3 tracts. This slowing was greatly exacerbated in the absence of RRM3, shown here to encode a 5 ,to 3 DNA helicase. Rrm3p-dependent fork progression was seen at a modified Chromosome VII-L telomere at the natural X-bearing Chromosome III-L telomere, and at Y-bearing telomeres. Loss of Rrm3p also resulted in replication fork pausing at specific sites in subtelomeric DNA, such as at inactive replication origins, and at internal tracts of C1–3A/TG1–3 DNA. The ATPase/helicase activity of Rrm3p was required for its role in telomeric and subtelomeric DNA replication. Because Rrm3p was telomere-associated in vivo, it likely has a direct role in telomere replication. [Key Words: Telomere; helicase; telomerase; replication; RRM3; yeast] Received February 7, 2002; revised version accepted April 10, 2002. Telomeres are the natural ends of eukaryotic chromo- Because conventional DNA polymerases cannot repli- somes. In most organisms, the very ends of chromo- cate the very ends of linear DNA molecules, special somes consist of simple repeated sequences. For ex- mechanisms are required to prevent the loss of terminal ample, Saccharomyces cerevisiae chromosomes end in DNA. -

BMC Biochemistry Biomed Central
BMC Biochemistry BioMed Central Methodology article Open Access Displacement affinity chromatography of protein phosphatase one (PP1) complexes Greg BG Moorhead*1, Laura Trinkle-Mulcahy2, Mhairi Nimick1, Veerle De Wever1, David G Campbell3, Robert Gourlay3, Yun Wah Lam2 and Angus I Lamond2 Address: 1Department of Biological Sciences, University of Calgary, 2500 University Dr. N.W. Calgary, AB T2N 1N4, Canada, 2Wellcome Trust Biocentre, MSI/WTB Complex, University of Dundee, Dundee, DD1 5EH, UK and 3MRC Protein Phosphorylation Unit, School of Life Sciences, University of Dundee, Dundee, Scotland DD1 5EH, UK Email: Greg BG Moorhead* - [email protected]; Laura Trinkle-Mulcahy - [email protected]; Mhairi Nimick - [email protected]; Veerle De Wever - [email protected]; David G Campbell - [email protected]; Robert Gourlay - [email protected]; Yun Wah Lam - [email protected]; Angus I Lamond - [email protected] * Corresponding author Published: 10 November 2008 Received: 8 May 2008 Accepted: 10 November 2008 BMC Biochemistry 2008, 9:28 doi:10.1186/1471-2091-9-28 This article is available from: http://www.biomedcentral.com/1471-2091/9/28 © 2008 Moorhead et al; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Abstract Background: Protein phosphatase one (PP1) is a ubiquitously expressed, highly conserved protein phosphatase that dephosphorylates target protein serine and threonine residues. PP1 is localized to its site of action by interacting with targeting or regulatory proteins, a majority of which contains a primary docking site referred to as the RVXF/W motif. -

Supplemental Material Uremic Toxin Indoxyl Sulfate Promotes Pro
Supplemental Material Uremic toxin indoxyl sulfate promotes pro-inflammatory macrophage activation via the interplay of OATB2B1 and Dll4-Notch signaling Potential mechanism for accelerated atherogenesis in chronic kidney disease Toshiaki Nakano, MD, PhD; Masanori Aikawa, MD, PhD, et al. Online materials and methods supplement The data, analytic methods, and study materials will be made available to other researchers upon request for purposes of reproducing the results or replicating the procedure. The data are available through https://aikawalabs.bwh.harvard.edu/CKD. Cell cultures Buffy coats were purchased from Research Blood Components, LLC (Boston, MA) and derived from de-identified healthy donors. The company recruits healthy donors under a New England Institutional Review Board-approved protocol for the Collection of White Blood for Research Purposes (NEIRB#04-144). We had no access to the information about donors. Human PBMC, isolated by density gradient centrifugation from buffy coats, were cultured in RPMI-1640 (Thermo Fisher Scientific, Waltham, MA) containing 5% human serum and 1% penicillin/streptomycin for 10 days to differentiate macrophages. In stimulation assays, confluent macrophages were rinsed extensively with PBS, starved for 24 hours in 1% serum media with or without 4% albumin (Sigma-Aldrich, St. Louis MO), and treated with indoxyl sulfate (Sigma-Aldrich) or tryptophan (Sigma-Aldrich). In inhibition assays, macrophages were starved for 24 hours and pretreated with TAK-242 (10 µmol/L, Cayman chemical, Ann Arbor, MI) for 1 hour, γ-Secretase inhibitor (DAPT, 10 µmol/L, Sigma-Aldrich) or DMSO (Sigma- Aldrich) for 6 hours; anti-human Dll4 blocking antibody (MHD4-46, 10 μg/mL) or isotype IgG for 24 hours; or Probenecid (10 mmol/L, Sigma-Aldrich), Rifampicin (100µM, Sigma-Aldrich) 1 or Cyclosporin A (10 µmol/L, Sigma-Aldrich) for 30 minutes as in previous reports 1-3 before stimulation with indoxyl sulfate. -

(12) United States Patent (10) Patent No.: US 9,574.223 B2 Cali Et Al
USO095.74223B2 (12) United States Patent (10) Patent No.: US 9,574.223 B2 Cali et al. (45) Date of Patent: *Feb. 21, 2017 (54) LUMINESCENCE-BASED METHODS AND 4,826,989 A 5/1989 Batz et al. PROBES FOR MEASURING CYTOCHROME 4,853,371 A 8/1989 Coy et al. 4,992,531 A 2f1991 Patroni et al. P450 ACTIVITY 5,035,999 A 7/1991 Geiger et al. 5,098,828 A 3/1992 Geiger et al. (71) Applicant: PROMEGA CORPORATION, 5,114,704 A 5/1992 Spanier et al. Madison, WI (US) 5,283,179 A 2, 1994 Wood 5,283,180 A 2f1994 Zomer et al. 5,290,684 A 3/1994 Kelly (72) Inventors: James J. Cali, Verona, WI (US); Dieter 5,374,534 A 12/1994 Zomer et al. Klaubert, Arroyo Grande, CA (US); 5,498.523 A 3, 1996 Tabor et al. William Daily, Santa Maria, CA (US); 5,641,641 A 6, 1997 Wood Samuel Kin Sang Ho, New Bedford, 5,650,135 A 7/1997 Contag et al. MA (US); Susan Frackman, Madison, 5,650,289 A T/1997 Wood 5,726,041 A 3/1998 Chrespi et al. WI (US); Erika Hawkins, Madison, WI 5,744,320 A 4/1998 Sherf et al. (US); Keith V. Wood, Mt. Horeb, WI 5,756.303 A 5/1998 Sato et al. (US) 5,780.287 A 7/1998 Kraus et al. 5,814,471 A 9, 1998 Wood (73) Assignee: PROMEGA CORPORATION, 5,876,946 A 3, 1999 Burbaum et al. 5,976,825 A 11/1999 Hochman Madison, WI (US) 6,143,492 A 11/2000 Makings et al. -

The Rho Gtpase Rif Signals Through IRTKS, Eps8 and WAVE2 To
© 2016. Published by The Company of Biologists Ltd | Journal of Cell Science (2016) 129, 2829-2840 doi:10.1242/jcs.179655 RESEARCH ARTICLE The Rho GTPase Rif signals through IRTKS, Eps8 and WAVE2 to generate dorsal membrane ruffles and filopodia Thankiah Sudhaharan1, Kai Ping Sem1, Hwi Fen Liew1, Yuan Hong Yu1, Wah Ing Goh1,2, Ai Mei Chou1 and Sohail Ahmed1,* ABSTRACT (Ahmed et al., 2010). Cdc42 is thought to activate and localize Rif induces dorsal filopodia but the signaling pathway responsible for IRSp53 (Disanza et al., 2006). As for Rif, it has been found to this has not been identified. We show here that Rif interacts with the partner the actin nucleators mDia1 and mDia2 in forming peripheral I-BAR family protein IRTKS (also known as BAIAP2L1) through its filopodia (Goh et al., 2011; Pellegrin and Mellor, 2005). I-BAR domain. Rif also interacts with Pinkbar (also known as The I-BAR family of proteins contain five members: IRSp53, BAIAP2L2) in N1E-115 mouse neuroblastoma cells. IRTKS and Rif IRTKS (insulin receptor tyrosine kinase substrate; also known as induce dorsal membrane ruffles and filopodia. Dominant-negative Rif BAIAP2L1 or BAI1-associated protein 2-like 1), MIM (missing in inhibits the formation of IRTKS-induced morphological structures, metastasis; also known as MTSS1), ABBA (actin-bundling protein and Rif activity is blocked in IRTKS-knockout (KO) cells. To further with BAIAP2 homology; also known as MTSS1L), and Pinkbar define the Rif–IRTKS signaling pathway, we identify Eps8 and (Planar intestinal-and kidney-specific BAR domain protein; also WAVE2 (also known as WASF2) as IRTKS interactors. -

A Proteome Approach Reveals Differences Between Fertile Women
Article A Proteome Approach Reveals Differences between Fertile Women and Patients with Repeated Implantation Failure on Endometrial Level–Does hCG Render the Endometrium of RIF Patients? Alexandra P. Bielfeld 1,†, Sarah Jean Pour 1,†, Gereon Poschmann 2, Kai Stühler 2,3, Jan-Steffen Krüssel 1 and Dunja M. Baston-Büst 1,* 1 Medical Center University of Düsseldorf, Department of OB/GYN and REI (UniKiD), Moorenstrasse 5, 40225 Düsseldorf, Germany; [email protected] (A.P.B.); [email protected] (S.J.P.); [email protected] (J.-S.K.) 2 Molecular Proteomics Laboratory, Biomedical Research Centre (BMFZ), Heinrich-Heine-University, Universitätsstrasse 1, 40225 Düsseldorf, Germany; [email protected] (G.P.); [email protected] (K.S.) 3 Institute for Molecular Medicine, University Hospital Düsseldorf, 40225 Düsseldorf, Germany * Correspondence: [email protected]; Tel.: +49-211-81-08110; Fax: +49-211-81-16787 † These authors have contributed equally. Received: 27 December 2018; Accepted: 17 January 2019; Published: 19 January 2019 Abstract: Background: The molecular signature of endometrial receptivity still remains barely understood, especially when focused on the possible benefit of therapeutical interventions and implantation-related pathologies. Therefore, the protein composition of tissue and isolated primary cells (endometrial stromal cells, ESCs) from endometrial scratchings of ART (Assisted Reproductive Techniques) patients with repeated implantation failure (RIF) was compared to volunteers with proven fertility during the time of embryo implantation (LH + 7). Furthermore, an analysis of the endometrial tissue of fertile women infused with human chorionic gonadotropin (hCG) was conducted. Methods: Endometrial samples (n = 6 RIF, n = 10 fertile controls) were split into 3 pieces: 1/3 each was frozen in liquid nitrogen, 1/3 fixed in PFA and 1/3 cultured. -

Rho Gtpases of the Rhobtb Subfamily and Tumorigenesis1
Acta Pharmacol Sin 2008 Mar; 29 (3): 285–295 Invited review Rho GTPases of the RhoBTB subfamily and tumorigenesis1 Jessica BERTHOLD2, Kristína SCHENKOVÁ2, Francisco RIVERO2,3,4 2Centers for Biochemistry and Molecular Medicine, University of Cologne, Cologne, Germany; 3The Hull York Medical School, University of Hull, Hull HU6 7RX, UK Key words Abstract Rho guanosine triphosphatase; RhoBTB; RhoBTB proteins constitute a subfamily of atypical members within the Rho fa- DBC2; cullin; neoplasm mily of small guanosine triphosphatases (GTPases). Their most salient feature 1This work was supported by grants from the is their domain architecture: a GTPase domain (in most cases, non-functional) Center for Molecular Medicine Cologne, the is followed by a proline-rich region, a tandem of 2 broad-complex, tramtrack, Deutsche Forschungsgemeinschaft, and the bric à brac (BTB) domains, and a conserved C-terminal region. In humans, Köln Fortune Program of the Medical Faculty, University of Cologne. the RhoBTB subfamily consists of 3 isoforms: RhoBTB1, RhoBTB2, and RhoBTB3. Orthologs are present in several other eukaryotes, such as Drosophi- 4 Correspondence to Dr Francisco RIVERO. la and Dictyostelium, but have been lost in plants and fungi. Interest in RhoBTB Phn 49-221-478-6987. Fax 49-221-478-6979. arose when RHOBTB2 was identified as the gene homozygously deleted in E-mail [email protected] breast cancer samples and was proposed as a candidate tumor suppressor gene, a property that has been extended to RHOBTB1. The functions of RhoBTB pro- Received 2007-11-17 Accepted 2007-12-16 teins have not been defined yet, but may be related to the roles of BTB domains in the recruitment of cullin3, a component of a family of ubiquitin ligases. -

Réseau Régulatoire De HDAC3 Pour Comprendre Les Mécanismes De Différenciation Et De Pathogenèse De Toxoplasma Gondii
THÈSE Pour obtenir le grade de DOCTEUR DE LA COMMUNAUTE UNIVERSITE GRENOBLE ALPES Spécialité : Physiologie-Physiopathologies-Pharmacologie Arrêté ministériel : 25 mai 2016 Présentée par « Fabien SINDIKUBWABO » Thèse dirigée par Mohamed-Ali HAKIMI, DR2, INSERM- Institute for Advanced Biosciences IAB, Grenoble Préparée au sein du Laboratoire Host-Pathogens interactions & Immunity to Infections. Dans l'École Doctorale Chimie et Sciences du vivant. Réseau régulatoire de HDAC3 pour comprendre les mécanismes de différenciation et de pathogenèse de Toxoplasma gondii Thèse soutenue publiquement le « 12 octobre 2017 », devant le jury composé de : M.ARTUR SCHERF Directeur de recherche Emérite et CNRS Ile de France Ouest et Nord, Rapporteur M. THIERRY LAGRANGE Directeur de recherche et CNRS Délégation Languedoc-Roussillon, Rapporteur M.SAADI KHOCHBIN Directeur de recherche Emérite et CNRS Délégation Alpes, Président et examinateur M. JEROME GOVIN Directeur de recherche et CEA Grenoble, Examinateur Acknowledgements I would like to express my sincere gratitude to my advisor, Dr. Hakimi Mohamed-Ali, who gave me the chance to do my PhD in his laboratory. He was always present and worked hard to train me and encouraged me to strive for success during these past four years. This work would not have been possible without his continuous support and advice in all the time of my research. My sincere thanks go also to Dr. Thierry Lagrange and Mr. Hassan Belrhali for their pertinent suggestions during my thesis. Many thanks to Dr.Saadi Khochbin, Dr. Artur Scherf, Dr. Thierry Lagrange and Dr. Jérôme Govin who, as thesis committee members, accepted to examine my work. I am thankful to the people in Hakimi laboratory who participated to my thesis: Dominique, Laurence, Alexandre, Céline, Hélène, Huan, Gabrielle, Dayana, Marie Pierre, Hervé, Tahir, Andrés, Christopher, Rose, Bastien, Aurélie, Séverine and Valeria. -

Critical Care Management and Future Research
JOURNAL OF EXPLORATORY RESEARCH IN PHARMACOLOGY CONTENTS 2018 3(3):71–91 Original Articles Induction of Amodiaquine Metabolism by Rifampicin Following Concurrent Administration in Healthy Volunteers Adebusuyi Akande Ademisoye, Julius Olugbenga Soyinka, Samuel Olanrewaju Olawoye, Sharon Iyobor Igbinoba, Samuel Anu Olowookere, Adelola Taiwo Ademisoye, Cyprian Ogbona Onyeji 71 Evaluation of Diagnostic Methods and Antimicrobial Susceptibility Pattern of Asymptomatic Bacteriuria Among Pregnant Women in Ashanti Region, Ghana Desmond O Acheampong, Michael K Afoakwah, Alex Boye, Richard Opoku, Godwin Kwakye-Nuako, Christian K Adokoh, Samuel A Baafi, Daniel Somuah 78 Review Article Luteolin: Anti-breast Cancer Effects and Mechanisms Ying Wang, Jing Wang, Xing Gong, Xinxuan Wen, Xinsheng Gu 85 Letter to the Editor Re: Evaluation of Diagnostic Methods and Antimicrobial Susceptibility Pattern of Asymptomatic Bacteriuria among Pregnant Women in Ashanti Region, Ghana Chenglong Wang, Jiaming Wang, Yingdan Gu, Danqing Pang, Dong Yin 91 Original Article Induction of Amodiaquine Metabolism by Rifampicin Following Concurrent Administration in Healthy Volunteers Adebusuyi Akande Ademisoye1, Julius Olugbenga Soyinka1*, Samuel Olanrewaju Olawoye1, Sharon Iyobor Igbinoba2, Samuel Anu Olowookere3, Adelola Taiwo Ademisoye1 and Cyprian Ogbona Onyeji1 1Department of Pharmaceutical Chemistry, Obafemi Awolowo University, Ile-Ife, Nigeria; 2Department of Clinical Pharmacy and Pharmacy Administration, Obafemi Awolowo University, Ile-Ife, Nigeria; 3Department of Community Health, College of Health Sciences, Obafemi Awolowo University, Ile-Ife, Nigeria Abstract Background and objective: Malaria and tuberculosis remain endemic in tropical regions and most often coexist, increasing the burden of malaria mortality due to unintended interactions of the co-administered drugs employed in the management of the infections. Rifampicin (RIF) and amodiaquine (ADQ) are likely to be administered con- currently in the treatment of patients with tuberculosis and malaria. -

Lawrence Berkeley National Laboratory Recent Work
Lawrence Berkeley National Laboratory Recent Work Title THE REDUCTIVE PENTOSE PHOSPHATE CYCLE AND ITS REGULATION Permalink https://escholarship.org/uc/item/5576992j Author Bassham, James A. Publication Date 1977-02-01 eScholarship.org Powered by the California Digital Library University of California u u d.U -4 7 0 9 ~ J 0 Submitted to Encyclopedia of Plant LBL-6123 Physiology Preprint C..\ '~- THE REDUCTIVE PENTOSE PHOSPHATE CYCLE AND ITS REGULATION James A. Bas sham February 19 77 / Prepared for the U. S. Energy Research and Development Administration under Contract W -7405-ENG-48 For Reference Not to be taken from this room DISCLAIMER This document was prepared as an account of work sponsored by the United States Government. While this document is believed to contain correct information, neither the United States Government nor any agency thereof, nor the Regents of the University of California, nor any of their employees, makes any warranty, express or implied, or assumes any legal responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by its trade name, trademark, manufacturer, or otherwise, does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof, or the Regents of the University of California. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof or the Regents of the University of California. -

Publ Prelims Issue Ge3901 Page 1
List of abbreviations A – adenosine Myr – million years A – absorbance (1 cm) N – any nucleoside aa – amino acid(s) NAD L nicotinamide-adenine dinucleotide – Ab – antibody(ies) NADH K and its reduced form Ad – adenovirus Nm – neomycin AdoMet (or SAM) – S-adenosylmethionine nt – nucleotide(s) AMV – avian myeloblastosis virus o, O – operator Ap – ampicillin oligo – oligodeoxyribonucleotide bGal – b-galactosidase ONPG – o-nitrophenyl b-d-galactopyranoside bp – base pair(s) ORF – open reading frame BSA – bovine serum albumin ori – origin(s) of DNA replication C – cytidine p – plasmid cAMP – cyclic adenosine 3∞,5∞-monophosphate p, P – promoter CAT – Cm acetyltransferase PA – polyacrylamide cat – gene encoding CAT PAGE – PA-gel electrophoresis ccc – covalently closed circular PEG – poly(ethylene glycol) cDNA – DNA complementary to RNA pfu – plaque-forming unit(s) CHO – Chinese hamster ovary Pi – inorganic phosphate CIAP – calf intestinal alkaline phosphatase Pipes – 1,4-piperazinediethanesulfonic acid Cm – chloramphenicol PMSF – phenylmethylsulfonyl fluoride cp – chloroplast PolIk – Klenow (large) fragment of E. coli DNA cpm – counts per minute polymerase I d – deoxyribo PPi – inorganic pyrophosphate D – deletion PPO – 2,5-diphenyloxazole dd – dideoxyribo R – (superscript) resistance/resistant DMSO – dimethylsulfoxide R – purine (or restriction) DNase – deoxyribonuclease RBS – ribosome-binding site(s) dNTP – deoxyribonucleoside triphosphate rDNA – DNA coding for rRNA ds – double strand(ed) re- – recombinant DTT – dithiothreitol RFLP – restriction-fragment -

Identification of Lysine Methylation in the Core Gtpase Domain by Gomadscan
RESEARCH ARTICLE Identification of lysine methylation in the core GTPase domain by GoMADScan 1☯ 2☯ 1,3☯ 2 Hirofumi Yoshino , Guowei YinID , Risa Kawaguchi , Konstantin I. Popov , Brenda Temple4, Mika Sasaki1, Satoshi Kofuji1, Kara Wolfe1, Kaori Kofuji1, Koichi Okumura1, Jaskirat Randhawa1, Akshiv Malhotra1, Nazanin Majd5, Yoshiki Ikeda1, Hiroko Shimada1, Emily Rose Kahoud6, Sasson Haviv6, Shigeki Iwase7, John M. Asara6, 2 1,8,9,10 Sharon L. Campbell *, Atsuo T. SasakiID * 1 Division of Hematology and Oncology, Department of Internal Medicine, University of Cincinnati College of Medicine, Cincinnati, Ohio, United States of America, 2 Department of Biochemistry and Biophysics and Lineberger Comprehensive Cancer Center, University of North Carolina School of Medicine, Chapel Hill, a1111111111 North Carolina, United States of America, 3 Department of Computational Biology and Medical Sciences, a1111111111 Graduate School of Frontier Sciences, University of Tokyo, Kashiwa, Chiba, Japan, 4 University of North a1111111111 Carolina, R. L. Juliano Structural Bioinformatics Core Facility, Chapel Hill, North Carolina, United States of a1111111111 America, 5 Department of Neurology, University of Cincinnati College of Medicine, Cincinnati, Ohio, United a1111111111 States of America, 6 Harvard Medical School, Department of Medicine and Beth Israel Deaconess Medical Center, Division of Signal Transduction, Boston, Massachusetts, United States of America, 7 Department of Human Genetics, University of Michigan, 5815 Medical Science II, Ann Arbor, Michigan, United States of America, 8 Department of Cancer Biology, University of Cincinnati College of Medicine, Ohio, United States of America, 9 Department of Neurosurgery, Brain Tumor Center at UC Gardner Neuroscience Institute, Cincinnati, Ohio, United States of America, 10 Institute for Advanced Biosciences, Keio University, Tsuruoka, OPEN ACCESS Yamagata, Japan Citation: Yoshino H, Yin G, Kawaguchi R, Popov ☯ These authors contributed equally to this work.