Sorting the Alphabet Soup of Renal Pathology: a Review
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Contemporary Concepts and Imaging Findings in Paediatric Cystic Kidney Disease, P
Contemporary concepts and imaging findings in paediatric cystic kidney disease, p. 65-79 HR VOLUME 3 | ISSUE 2 J Urogenital Imaging Review Contemporary concepts and imaging findings in paediatric cystic kidney disease Vasiliki Dermentzoglou, Virginia Grigoraki, Maria Zarifi Department of Radiology, Agia Sophia Children's Hospital, Athens, Greece Submission: 31/1/2018 | Acceptance: 27/5/2018 Abstract The purpose of this article is to review the renal cyst- cystic tumours. Imaging plays an important role, as ic diseases in children with regard to classification, it helps to detect and characterise many of the cyst- genetic background, antenatal and postnatal ultra- ic diseases based primarily on detailed sonographic sonographic appearances and evolution of findings analysis. Diagnosis can be achieved in many condi- in childhood. Numerous classifications exist, even tions during foetal life with ultrasound (US) and in though the prevailing one divides cystic diseases in selected cases with foetal magnetic resonance imag- hereditary and non-hereditary. Contemporary data ing (MRI). After birth, combined use of conventional are continuously published for most of the sub-cat- and high-resolution US allows detailed definition of egories. Genetic mutations at the level of primary the extent and evolution of kidney manifestations. cilia are considered a causative factor for many re- Appropriate monitoring with US seems crucial for nal cystic diseases which are now included in the patients’ management. In selected cases (e.g. hepa- spectrum of ciliopathies. Genetic mapping has doc- tobiliary disease, cystic tumours) primarily MRI and umented gene mutations in cystic diseases that are occasionally computed tomography (CT) are valua- generally considered non-hereditary, as well as in ble diagnostic tools. -

Renal Relevant Radiology: Use of Ultrasonography in Patients with AKI
Renal Relevant Radiology: Use of Ultrasonography in Patients with AKI Sarah Faubel,* Nayana U. Patel,† Mark E. Lockhart,‡ and Melissa A. Cadnapaphornchai§ Summary As judged by the American College of Radiology Appropriateness Criteria, renal Doppler ultrasonography is the most appropriate imaging test in the evaluation of AKI and has the highest level of recommendation. Unfortunately, nephrologists are rarely specifically trained in ultrasonography technique and interpretation, and *Division of Internal Medicine, Nephrology, important clinical information obtained from renal ultrasonography may not be appreciated. In this review, the University of Colorado strengths and limitations of grayscale ultrasonography in the evaluation of patients with AKI will be discussed and Denver Veterans with attention to its use for (1) assessment of intrinsic causes of AKI, (2) distinguishing acute from chronic kidney Affairs Medical Center, 3 Denver, Colorado; diseases, and ( ) detection of obstruction. The use of Doppler imaging and the resistive index in patients with † AKI will be reviewed with attention to its use for (1) predicting the development of AKI, (2) predicting the Department of 3 Radiology and prognosis of AKI, and ( ) distinguishing prerenal azotemia from intrinsic AKI. Finally, pediatric considerations in §Department of Internal the use of ultrasonography in AKI will be reviewed. Medicine and Clin J Am Soc Nephrol 9: 382–394, 2014. doi: 10.2215/CJN.04840513 Pediatrics, Nephrology, University of Colorado Denver, Denver, Colorado; and Introduction structures on ultrasonography images. The renal cap- ‡Department of Renal ultrasonography is typically the most appro- sule consists of thin fibrous tissue, which is next to Radiology, University of priate and useful radiologic test in the evaluation of fat, and thus the kidney often appears to be surroun- Alabama at Birmingham, patients with AKI (1). -

10 Renal Infarction 13 Renal
1 Kidneys and Adrenals P. Hein, U. Lemke, P. Asbach Renal Anomalies 1 Angiomyolipoma 47 Medullary Sponge Kidney 5 Hypovascular Renal Cell Accessory Renal Arteries 8 Carcinoma 50 Renal Artery Stenosis (RAS) 10 Oncocytoma 52 Renal Infarction 13 Renal Cell Carcinoma 54 Renal VeinThrombosis 16 Cystadenoma and Cystic Renal Renal Trauma/Injuries 19 Cell Carcinoma 59 Acute Pyelonephritis 23 Renal Lymphoma 62 Chronic Pyelonephritis 26 Renal Involvement in Xanthogranulomatous Phakomatoses 65 Pyelonephritis 29 Kidney Transplantation I 67 Pyonephrosis 31 Kidney Transplantation II 70 Renal Abscess 33 Adrenocortical Hyperplasia 73 Renal Tuberculosis 36 Adrenal Adenoma 76 Renal Cysts I Adrenocortical Carcinoma 81 (Simple, Parapelvic, Cortical) 38 Pheochromocytoma 85 Renal Cysts II Adrenal Metastasis 88 (Complicated, Atypical) 41 Adrenal Calcification 91 Polycystic Kidney Disease 44 AdrenalCysts 93 2 The Urinary Tract P. Asbach, D. Beyersdorff Ureteral Duplication Anomalies.. 96 BladderDiverticula 127 Megaureter 99 Urothelial Carcinoma Ureterocele 101 oftheBIadder 129 Anomalies of the Male Urethral Strictures 133 Ureteropelvicjunction 103 Female Urethral Pathology 135 Vesicoureteral Reflux (VUR) 106 Vesicovaginal and Vesicorectal Acute Urinary Obstruction 109 Fistulas 138 Chronic Urinary Obstruction 112 The Postoperative Lower Retroperitoneal Fibrosis 115 Urinary Tract 140 Urolithiasis 118 BladderRupture 142 Ureteral Injuries 122 Urethral and Penile Trauma 145 Urothelial Carcinoma of the Renal Peivis and Ureter 124 Bibliografische Informationen digitalisiert durch http://d-nb.info/987804278 gescannt durch Contents 3 The Male Cenitals U. Lemke. D. Beyersdorff, P. Asbach Scrotal Anatomy 148 Varicocele 169 Hydrocele 151 Benign Prostatic Hyperplasia Testicular and Epididymal Cysts 153 (BPH) 171 Testicular Microlithiasis 155 Prostatitis 174 Epididymoorchitis 157 Prostate Cancer 176 Testicular Tumors 160 Penile Cavernosal Fibrosis 179 Testicular Torsion 164 Peyronie Disease 181 Testicular Trauma 166 Penile Malignancies 184 4 The Female Cenitals U. -

Autosomal Dominant Medullary Cystic Kidney Disease (ADMCKD)
Autosomal Dominant Medullary Cystic Kidney Disease (ADMCKD) Author: Doctor Antonio Amoroso1 Creation Date: June 2001 Scientific Editor: Professor Francesco Scolari 1Servizio Genetica e Cattedra di Genetica, Istituto per l'infanzia burlo garofolo, Via dell'Istria 65/1, 34137 Trieste, Italy. [email protected] Abstract Keywords Disease name Synonyms Diagnostic criteria Differential diagnosis Prevalence Clinical description Management Etiology Genetic counseling References Abstract Autosomal dominant medullary cystic kidney disease (ADMCKD) belongs, together with nephronophthisis (NPH), to a heterogeneous group of inherited tubulo-interstitial nephritis, termed NPH-MCKD complex. The disorder, usually first seen clinically at an average age of 28 years, is characterized by structural defects in the renal tubules, leading to a reduction of the urine–concentrating ability and decreased sodium conservation. Clinical onset and course of ADMCKD are insidious. The first sign is reduced urine– concentrating ability. Clinical symptoms appear when the urinary concentrating ability is markedly reduced, producing polyuria. Later in the course, the clinical findings reflect the progressive renal insufficiency (anemia, metabolic acidosis and uremic symptoms). End-stage renal disease typically occurs in the third-fifth decade of life or even later. The pathogenesis of ADMCKD is still obscure and how the underlying genetic abnormality leads to renal disease is unknown. ADMCKD is considered to be a rare disease. Until 2000, 55 affected families had been described. There is no specific therapy for ADMCKD other than correction of water and electrolyte imbalances that may occur. Dialysis followed by renal transplantation is the preferred approach for end-stage renal failure. Keywords Autosomal dominant medullary cystic disease, medullary cysts, nephronophthisis, tubulo-interstitial nephritis Disease name Diagnostic criteria Autosomal dominant medullary cystic kidney The renal presentation of MCKD is relatively disease (ADMCKD) non-specific. -

Management of the Incidental Renal Mass on CT: a White Paper of the ACR Incidental Findings Committee
ORIGINAL ARTICLE Management of the Incidental Renal Mass on CT: A White Paper of the ACR Incidental Findings Committee Brian R. Herts, MDa, Stuart G. Silverman, MDb, Nicole M. Hindman, MDc, Robert G. Uzzo, MDd, Robert P. Hartman, MDe, Gary M. Israel, MD f, Deborah A. Baumgarten, MD, MPHg, Lincoln L. Berland, MDh, Pari V. Pandharipande, MD, MPHi Abstract The ACR Incidental Findings Committee (IFC) presents recommendations for renal masses that are incidentally detected on CT. These recommendations represent an update from the renal component of the JACR 2010 white paper on managing incidental findings in the adrenal glands, kidneys, liver, and pancreas. The Renal Subcommittee, consisting of six abdominal radiologists and one urologist, developed this algorithm. The recommendations draw from published evidence and expert opinion and were finalized by informal iterative consensus. Each flowchart within the algorithm describes imaging features that identify when there is a need for additional imaging, surveillance, or referral for management. Our goal is to improve quality of care by providing guidance for managing incidentally detected renal masses. Key Words: Kidney, renal, small renal mass, cyst, Bosniak classification, incidental finding J Am Coll Radiol 2017;-:---. Copyright Ó 2017 American College of Radiology OVERVIEW OF THE ACR INCIDENTAL Findings Committee (IFC) generated its first white paper FINDINGS PROJECT in 2010, addressing four algorithms for managing inci- The core objectives of the Incidental Findings Project are dental pancreatic, adrenal, kidney, and liver findings [1]. to (1) develop consensus on patient characteristics and imaging features that are required to characterize an THE CONSENSUS PROCESS: THE INCIDENTAL incidental finding, (2) provide guidance to manage such RENAL MASS ALGORITHM findings in ways that balance the risks and benefits to The current publication represents the first revision of the patients, (3) recommend reporting terms that reflect the IFC’s recommendations on incidental renal masses. -

A Cross Sectional Study Ofrenal Involvement in Tuberous Sclerosis
480 Med Genet 1996;33:480-484 A cross sectional study of renal involvement in tuberous sclerosis J Med Genet: first published as 10.1136/jmg.33.6.480 on 1 June 1996. Downloaded from J A Cook, K Oliver, R F Mueller, J Sampson Abstract There are two characteristic types of renal Renal disease is a frequent manifestation involvement in persons with TSC. (1) An- oftuberous sclerosis (TSC) and yet little is giomyolipomas. These are benign neoplasms known about its true prevalence or natural composed of mature adipose tissue, thick history. We reviewed the notes of 139 walled blood vessels, and smooth muscle in people with TSC, who had presented with- varying proportions. In the general population out renal symptoms, but who had been they are a rare finding affecting predominantly investigated by renal ultrasound. In- women (80%) in the third to fifth decade. formation on the frequency, type, and About 50% of people with angiomyolipomas symptomatology of renal involvement was have no stigmata of TSC and usually have retrieved. a large, single angiomyolipoma.6 In TSC the The prevalence ofrenal involvement was angiomyolipomas tend to be small, multiple, 61%. Angiomyolipomas were detected in and bilateral.7 Stillwell et al7 suggested that 49%, renal cysts in 32%, and renal car- there was an increase in the prevalence of cinoma in 2-2%. The prevalence of an- angiomyolipomas with age but only a limited giomyolipoma was positively correlated number of persons with TSC were studied. with age, compatible with a two hit aeti- (2) Renal cysts. These have a characteristic ology. -

ACR Appropriateness Criteria: Indeterminate Renal Mass
Revised 2020 American College of Radiology ACR Appropriateness Criteria® Indeterminate Renal Mass Variant 1: Indeterminate renal mass. No contraindication to either iodinated CT contrast or gadolinium- based MR intravenous contrast. Initial imaging. Procedure Appropriateness Category Relative Radiation Level US abdomen with IV contrast Usually Appropriate O MRI abdomen without and with IV contrast Usually Appropriate O CT abdomen without and with IV contrast Usually Appropriate ☢☢☢☢ US kidneys retroperitoneal May Be Appropriate O MRI abdomen without IV contrast May Be Appropriate O CT abdomen with IV contrast May Be Appropriate ☢☢☢ CT abdomen without IV contrast May Be Appropriate ☢☢☢ CTU without and with IV contrast May Be Appropriate ☢☢☢☢ Arteriography kidney Usually Not Appropriate ☢☢☢ Radiography intravenous urography Usually Not Appropriate ☢☢☢ Biopsy renal mass Usually Not Appropriate Varies MRU without and with IV contrast Usually Not Appropriate O Variant 2: Indeterminate renal mass. Contraindication to both iodinated CT and gadolinium-based MR intravenous contrast. Initial imaging. Procedure Appropriateness Category Relative Radiation Level US abdomen with IV contrast Usually Appropriate O US kidneys retroperitoneal Usually Appropriate O MRI abdomen without IV contrast Usually Appropriate O CT abdomen without IV contrast May Be Appropriate ☢☢☢ Arteriography kidney Usually Not Appropriate ☢☢☢ Radiography intravenous urography Usually Not Appropriate ☢☢☢ Biopsy renal mass Usually Not Appropriate Varies MRI abdomen without and with IV contrast Usually Not Appropriate O MRU without and with IV contrast Usually Not Appropriate O CT abdomen with IV contrast Usually Not Appropriate ☢☢☢ CT abdomen without and with IV contrast Usually Not Appropriate ☢☢☢☢ CTU without and with IV contrast Usually Not Appropriate ☢☢☢☢ ACR Appropriateness Criteria® 1 Indeterminate Renal Mass Variant 3: Indeterminate renal mass. -

Renal Papillary Necrosis in Infancy PETER HUSBAND* and K
Arch Dis Child: first published as 10.1136/adc.48.2.116 on 1 February 1973. Downloaded from Archives of Disease in Childhood, 1973, 48, 116. Renal papillary necrosis in infancy PETER HUSBAND* and K. A. HOWLETT From the Departments of Paediatrics and Radiology, Charing Cross Group of Hospitals, Fulham Hospital, London Husband, P., and Howlett, K. A. (1973). Archives of Disease in Childhood, 48, 116. Renal papillary necrosis in infancy. Two infants developed renal papillary necrosis after acute illnesses associated with dehydration. After a short oliguric phase there was a longer phase characterized by impaired urinary concen- tration, hyponatraemia, metabolic acidosis, and a raised blood urea. Intravenous urograms showed contrast collecting in the papillae, with subsequent sinus formation extending into the medulla. In one case impaired urinary concentration was still present 21 months after the initial illness. Renal papillary necrosis was originally described admitted to hospital 9 days later after he had been by Friedreich (1877) in a man aged 70 years. found in his cot, grey, with respiratory distress and a Since then the majority of further cases reported high temperature. 3 days before he had diarrhoea and have also been in adults. It has been thought to vomiting for 24 hours but subsequently tolerated feeds be uncommon and usually to have a fatal outcome of full cream Cow and Gate milk, 180 ml 5 times a day. He was again extremely ill: temperature 40 3 °C, pale, copyright. in infancy and childhood (Davies, Kennedy, and cyanosed with a rapid respiratory rate, but not dehydra- Roberts, 1969). In the newborn infant renal ted. -

Irish Rare Kidney Disease Network (IRKDN)
Irish Rare kidney Disease Network (IRKDN) Others Cork University Mater, Waterford University Dr Liam Plant Hospital Galway Dr Abernathy University Hospital Renal imaging Dr M Morrin Prof Griffin Temple St and Crumlin Beaumont Hospital CHILDRENS Hospital Tallaght St Vincents Dr Atiff Awann Rare Kidney Disease Clinic Hospital University Hospital Prof Peter Conlon Dr Lavin Prof Dr Holian Little Renal pathology Lab Limerick University Dr Dorman and Hospital Dr Doyle Dr Casserly Patient Renal Council Genetics St James Laboratory Hospital RCSI Dr Griffin Prof Cavaller MISION Provision of care to patients with Rare Kidney Disease based on best available medical evidence through collaboration within Ireland and Europe Making available clinical trials for rare kidney disease to Irish patients where available Collaboration with other centres in Europe treating rare kidney disease Education of Irish nephrologists on rare Kidney Disease. Ensuring a seamless transition of children from children’s hospital with rare kidney disease to adult centres with sharing of knowledge of rare paediatric kidney disease with adult centres The provision of precise molecular diagnosis of patients with rare kidney disease The provision of therapeutic plan based on understanding of molecular diagnosis where available Development of rare disease specific registries within national renal It platform ( Emed) Structure Beaumont Hospital will act as National rare Kidney Disease Coordinating centre working in conjunction with a network of Renal unit across the country -
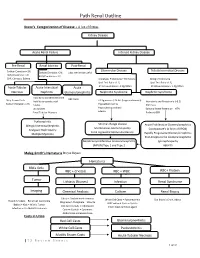
Path Renal Outline
Path Renal Outline Krane’s Categorization of Disease + A lot of Extras Kidney Disease Acute Renal Failure Intrinsic Kidney Disease Pre‐Renal Renal Intrinsic Post‐Renal Sodium Excretion <1% Glomerular Disease Tubulointerstitial Disease Sodium Excretion < 1% Sodium Excretion >2% Labs aren’t that useful BUN/Creatinine > 20 BUN/Creatinine < 10 CHF, Cirrhosis, Edema Urinalysis: Proteinuria + Hematuria Benign Proteinuria Spot Test Ratio >1.5, Spot Test Ratio <1.5, Acute Tubular Acute Interstitial Acute 24 Urine contains > 2.0g/24hrs 24 Urine contains < 1.0g/24hrs Necrosis Nephritis Glomerulonephritis Nephrotic Syndrome Nephritic Syndrome Inability to concentrate Urine RBC Casts Dirty Brown Casts Inability to secrete acid >3.5g protein / 24 hrs (huge proteinuria) Hematuria and Proteinuria (<3.5) Sodium Excretion >2% Edema Hypoalbuminemia RBC Casts Hypercholesterolemia Leukocytes Salt and Water Retention = HTN Focal Tubular Necrosis Edema Reduced GFR Pyelonephritis Minimal change disease Allergic Interstitial Nephritis Acute Proliferative Glomerulonephritis Membranous Glomerulopathy Analgesic Nephropathy Goodpasture’s (a form of RPGN) Focal segmental Glomerulosclerosis Rapidly Progressive Glomerulonephritis Multiple Myeloma Post‐Streptococcal Glomerulonephritis Membranoproliferative Glomerulonephritis IgA nephropathy (MPGN) Type 1 and Type 2 Alport’s Meleg‐Smith’s Hematuria Break Down Hematuria RBCs Only RBC + Crystals RBC + WBC RBC+ Protein Tumor Lithiasis (Stones) Infection Renal Syndrome Imaging Chemical Analysis Culture Renal Biopsy Calcium -

Acute Tubular Necrosıs After Nephrectomy: Case Presentatıon
Case Report JOJ Case Stud Volume 7 Issue 1 - May 2018 Copyright © All rights are reserved by Ebru Canakci DOI: 10.19080/JOJCS.2018.07.555703 Acute Tubular Necrosıs after Nephrectomy: Case Presentatıon Ebru Canakci1*, Ahmet Karatas2, Ahmet Gultekin1, Zubeyir Cebeci1, Ilker Coskun1 and Anıl Kılınc1 1Department of Anaesthesiology and Reanimation, Ordu University, Turkey 2Department of Internal Medicine & Nephrology, Ordu University, Turkey Submission: May 07, 2018; Published: May 18, 2018 *Corresponding author: Ebru Canakci, Faculty of Medicine, Department of Anaesthesiology and Reanimation, Ordu University, Ordu, Turkey, Email: Abstract ATN, contrary to prerenal azotemia, is not immediately cured upon the recovery of renal perfusion. In its severe form, renal hypoperfusion leads Acute renal failure (ARF) has a clinical presentation with declining renal function and glomerular filtration rate within hours-days. Ischemic trauma, severe hypovolemia, sepsis and severe burns. Acute kidney injury (AKI) is one of the frequently encountered causes of morbidity and mortalityto bilateral in renal hospitals. cortical The necrosis aim of this and study irreversible is to present renal the insufficiency. case with ATN Ischemic after major ATN often surgery develops and subsequent as a result permanent of major surgical kidney injuryintervention, in light of the information from the literature. Keywords: Nephrectomy; Acute Tubular Necrosis; Hemodialysis Introductıon and Objectıve to endogenous or exogenous toxins. Toxins cause intrarenal Acute renal failure (ARF) has a clinical presentation with vasoconstriction, direct tubular toxicity and/or intratubular obstruction and thus lead to ARF [4]. Acute kidney injury (AKI) hours-days. Although there are several differences in the declining renal function and glomerular filtration rate within is one of the frequently encountered causes of morbidity and mortality in hospitals. -

Effects of Bodybuilding Supplements on the Kidney
Ali et al. BMC Nephrology (2020) 21:164 https://doi.org/10.1186/s12882-020-01834-5 RESEARCH ARTICLE Open Access Effects of bodybuilding supplements on the kidney: A population-based incidence study of biopsy pathology and clinical characteristics among middle eastern men Alaa Abbas Ali1, Safaa E. Almukhtar2†, Dana A. Sharif3†, Zana Sidiq M. Saleem4†, Dana N. Muhealdeen1 and Michael D. Hughson1* Abstract Background: The incidence of kidney diseases among bodybuilders is unknown. Methods: Between January 2011 and December 2019, the Iraqi Kurdistan 15 to 39 year old male population averaged 1,100,000 with approximately 56,000 total participants and 25,000 regular participants (those training more than 1 year). Annual age specific incidence rates (ASIR) with (95% confidence intervals) per 100,000 bodybuilders were compared with the general age-matched male population. Results: Fifteen male participants had kidney biopsies. Among regular participants, diagnoses were: focal segmental glomerulosclerosis (FSGS), 2; membranous glomerulonephritis (MGN), 2; post-infectious glomeruonephritis (PIGN), 1; tubulointerstitial nephritis (TIN), 1; and nephrocalcinosis, 2. Acute tubular necrosis (ATN) was diagnosed in 5 regular participants and 2 participants training less than 1 year. Among regular participants, anabolic steroid use was self- reported in 26% and veterinary grade vitamin D injections in 2.6%. ASIR for FSGS, MGN, PIGN, and TIN among regular participants was not statistically different than the general population. ASIR of FSGS adjusted for anabolic steroid use was 3.4 (− 1.3 to 8.1), a rate overlapping with FSGS in the general population at 2.0 (1.2 to 2.8). ATN presented as exertional muscle injury with myoglobinuria among new participants.