Molecular Model Building
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pre-Activity Quiz Answer Key (Pdf)
Name: ____________________________________________ Date: ___________________ Class: ________________________ Pre-Activity Quiz Answer Key 1. Consider a molecule of carbon monoxide (C O): A. Do you think the electrons in the triple bond pull closer to the C atom or the O atom, or are they equally shared? Use the concept of electronegativity to explain your response. Answer: They are closer to the O atom. Using the electronegativity scale from a textbook, the result is a polar covalent bond shifted towards the O atom. B. Is the bond polar or non-polar? Polar 2. In today’s engineering challenge, you will sketch out Lewis dot diagrams for various molecules and polyatomic ions. Then you will construct each molecule using a molecular model kit. The kits contain three different representations: colored balls, short sticks and long flexible springs. A. Each colored ball corresponds to a different atom. How can you determine which color to use for each atom? Use the reference sheet that comes with the kit. B. For what bond type do you think the short sticks are used? Covalent bonds C. If you were to build a triple bond, what would you use to represent a triple bond and how many would you use? Use springs, three springs 3. You will become familiar with different geometries of simple molecules. A. Name the theory used to predict molecular shapes of these molecules? VSEPR theory Molecular Models and 3-D Printing Activity—Pre-Activity Quiz Answer Key 1 Name: ____________________________________________ Date: ___________________ Class: ________________________ B. What if a molecule contains a central atom bonded to two identical outer atoms with the central atom surrounded by a lone pair of electrons? Name the geometry of this molecule. -
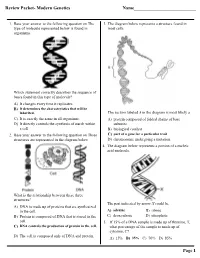
Review Packet- Modern Genetics Name___Page 1
Review Packet- Modern Genetics Name___________________________ 1. Base your answer to the following question on The 3. The diagram below represents a structure found in type of molecule represented below is found in most cells. organisms. Which statement correctly describes the sequence of bases found in this type of molecule? A) It changes every time it replicates. B) It determines the characteristics that will be inherited. The section labeled A in the diagram is most likely a C) It is exactly the same in all organisms. A) protein composed of folded chains of base D) It directly controls the synthesis of starch within subunits a cell. B) biological catalyst 2. Base your answer to the following question on Three C) part of a gene for a particular trait structures are represented in the diagram below. D) chromosome undergoing a mutation 4. The diagram below represents a portion of a nucleic acid molecule. What is the relationship between these three structures? The part indicated by arrow X could be A) DNA is made up of proteins that are synthesized in the cell. A) adenine B) ribose B) Protein is composed of DNA that is stored in the C) deoxyribose D) phosphate cell. 5. If 15% of a DNA sample is made up of thymine, T, C) DNA controls the production of protein in the cell. what percentage of the sample is made up of cytosine, C? D) The cell is composed only of DNA and protein. A) 15% B) 35% C) 70% D) 85% Page 1 6. Base your answer to the following question on Which 10.The diagram below represents genetic material. -

Application of X-Ray Diffraction Methods and Molecular Mechanics Simulations to Structure Determination and Cotton Fiber Analysis
University of New Orleans ScholarWorks@UNO University of New Orleans Theses and Dissertations Dissertations and Theses 12-19-2008 Application of X-ray Diffraction Methods and Molecular Mechanics Simulations to Structure Determination and Cotton Fiber Analysis Zakhia Moore University of New Orleans Follow this and additional works at: https://scholarworks.uno.edu/td Recommended Citation Moore, Zakhia, "Application of X-ray Diffraction Methods and Molecular Mechanics Simulations to Structure Determination and Cotton Fiber Analysis" (2008). University of New Orleans Theses and Dissertations. 888. https://scholarworks.uno.edu/td/888 This Dissertation is protected by copyright and/or related rights. It has been brought to you by ScholarWorks@UNO with permission from the rights-holder(s). You are free to use this Dissertation in any way that is permitted by the copyright and related rights legislation that applies to your use. For other uses you need to obtain permission from the rights-holder(s) directly, unless additional rights are indicated by a Creative Commons license in the record and/ or on the work itself. This Dissertation has been accepted for inclusion in University of New Orleans Theses and Dissertations by an authorized administrator of ScholarWorks@UNO. For more information, please contact [email protected]. Application of X-ray Diffraction Methods and Molecular Mechanics Simulations to Structure Determination and Cotton Fiber Analysis A Dissertation Submitted to the Graduate Faculty of the University of New Orleans in partial fulfillment of the Requirements for the Degree of Doctor of Philosophy in the Department of Chemistry by Zakhia Moore B.S., Xavier University, 2000 M.S., University of New Orleans, 2005 December, 2008 ACKNOWLEDGEMENTS Giving all glory to God for His many blessings, whom without I could not have completed this long journey. -

IAPS Act 15-17 Section Preview of the Teachers Edition.Pdf
Section Preview of the Teacher’s Edition for The Chemistry of Materials, Issues and Physical Science, 2nd Edition Activities 15-17 Suggested student responses and answer keys have been blocked out so that web-savvy students do not find this page and have access to answers. To experience a complete activity please request a sample found in the footer at lab-aids.com Families of Elements 15 4 s 0 2 n - o t si o es 50 te s -minu N O I T I N A ACTIVITY OVERVIEW V E S T I G Students compare physical and chemical properties of 13 elements and sort them into groups based on common properties. They then compare their classifications with groups—or families—of elements as defined by scientists and displayed in the Periodic Table of the Elements. KEY CONCEPTS AND PROCESS SKILLS (with correlation to NSE 5–8 Content Standards) 1. Elements have characteristic physical and chemical properties. Substances often are placed in categories or groups if they react in similar ways; metals are an example of such a group. (PhysSci: 1) 2. Scientists classify elements into families, based on their properties. (PhysSci: 1) 3. Students develop descriptions based on evidence. (Inquiry: 1) KEY VOCABULARY atom atomic mass element family (of elements) metal Periodic Table of the Elements B-39 Activity 15 • Families of Elements MATERIALS AND ADVANCE PREPARATION For the teacher aluminum, iron, copper, and carbon samples from Activity 14, “Physical and Chemical Properties of Materials” 1 Transparency 15.1, “Information on Element Cards” 8 sets of 4 Element Family Cards -

Inorganic Chemistry for Dummies® Published by John Wiley & Sons, Inc
Inorganic Chemistry Inorganic Chemistry by Michael L. Matson and Alvin W. Orbaek Inorganic Chemistry For Dummies® Published by John Wiley & Sons, Inc. 111 River St. Hoboken, NJ 07030-5774 www.wiley.com Copyright © 2013 by John Wiley & Sons, Inc., Hoboken, New Jersey Published by John Wiley & Sons, Inc., Hoboken, New Jersey Published simultaneously in Canada No part of this publication may be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, mechanical, photocopying, recording, scanning or otherwise, except as permitted under Sections 107 or 108 of the 1976 United States Copyright Act, without either the prior written permis- sion of the Publisher, or authorization through payment of the appropriate per-copy fee to the Copyright Clearance Center, 222 Rosewood Drive, Danvers, MA 01923, (978) 750-8400, fax (978) 646-8600. Requests to the Publisher for permission should be addressed to the Permissions Department, John Wiley & Sons, Inc., 111 River Street, Hoboken, NJ 07030, (201) 748-6011, fax (201) 748-6008, or online at http://www.wiley. com/go/permissions. Trademarks: Wiley, the Wiley logo, For Dummies, the Dummies Man logo, A Reference for the Rest of Us!, The Dummies Way, Dummies Daily, The Fun and Easy Way, Dummies.com, Making Everything Easier, and related trade dress are trademarks or registered trademarks of John Wiley & Sons, Inc. and/or its affiliates in the United States and other countries, and may not be used without written permission. All other trade- marks are the property of their respective owners. John Wiley & Sons, Inc., is not associated with any product or vendor mentioned in this book. -

Molecular Model-Building by Computer
Molecular Model-building by Computer In which biochemists observe models of giant molecules as they are displayed on a screen by a computer and try to fold them into the shapes that they assume in nature by Cyrus Levinthal any problems of modern biology a large number of problems in biology computer to help gain such information are concerned with the detailed into which biologists have some insight about the structure of large biological M relation between biological but concerning which they cannot yet molecules. function and molecular structure. Some ask suitable questions in terms of molec- For the first half of this century the of the questions currently being asked ular structure. As they see such prob- metabolic and structural relations among will be completely answered only when lems more clearly, however, they in- the small molecules of the living cell one has an understanding of the struc- variably find an increasing need for were the principal concern of bio- ture of all the molecular components of structural information. In our laboratory chemists. The chemical reactions these a biological system and a knowledge of at the Massachusetts Institute of Tech- molecules undergo have been studied how they interact. There are, of course, nology we have recently started using a intensively. Such reactions are specifical- ly catalyzed by the large protein mole- cules called enzymes, many of which have now been purified and also studied. It is only within the past few years, however, that X-ray-diffraction techniques have made it possible to determine the molecular structure of such protein molecules. -

76 Chapt-24-Organic2
The Chemistry of Alkanes Physical Properties of Alkanes as molecular size increases so does the boiling point of the alkane increased size increased dispersion forces Alkanes Boiling Point ˚C H Methane CH 4 H C H -161.6 H H H Ethane C2H6 H C C H -88.6 H H Propane C3H8 CH3 (CH2)1 CH3 -42.1 Butane C4H10 CH3 (CH2)2 CH3 -0.5 Pentane C5H12 CH3 (CH2)3 CH3 36.1 hexane C6H14 CH3 (CH2)4 CH3 68.7 Chemical Reactions and Alkanes because the C-C and C-H bonds are relatively strong , the alkanes are fairly unreactive their inertness makes them valuable as lubricating materials and as backbone material in the construction of other hydrocarbons Combustion of Alkanes At high temperatures alkanes combust ΔH˚ CH4 + O2 CO2 + 2H2O -890.4 kJ C4H10 + 13/2O2 4CO2 + 5H2O -3119 kJ these reactions are all highly exothermic Halogenation of Alkanes at temperatures above 100 ˚C CH4 + Cl2 CH3Cl + HCl chloromethane CH3Cl + Cl2 CH2Cl2 + HCl dichloromethane CH2Cl2 + Cl2 CHCl3 + HCl trichloromethane chloroform CHCl3 + Cl2 CCl4 + HCl tetrachloromethane Carbon tetrachloride Mechanism for Halogenation of Methane CH4 + Cl2 CH3Cl + HCl hν Cl2 • Cl + • Cl hν: energy required to break the Cl-Cl bond • Cl H H C H H • Cl is very reactive and able to attack the C-H bond Mechanism for Halogenation of Methane CH4 + Cl2 CH3Cl + HCl CH4 + • Cl • CH3 + HCl H Cl H • C H H Mechanism for Halogenation of Methane CH4 + Cl2 CH3Cl + HCl • CH3 + Cl2 CH3 Cl + • Cl Cl Cl H • Cl • C H H H Cl C H H Mechanism for Halogenation of Methane Cl2 • Cl + • Cl chlorine free radical CH4 + • Cl • CH3 + HCl -

Standard Thermodynamic Properties of Chemical
STANDARD THERMODYNAMIC PROPERTIES OF CHEMICAL SUBSTANCES ∆ ° –1 ∆ ° –1 ° –1 –1 –1 –1 Molecular fH /kJ mol fG /kJ mol S /J mol K Cp/J mol K formula Name Crys. Liq. Gas Crys. Liq. Gas Crys. Liq. Gas Crys. Liq. Gas Ac Actinium 0.0 406.0 366.0 56.5 188.1 27.2 20.8 Ag Silver 0.0 284.9 246.0 42.6 173.0 25.4 20.8 AgBr Silver(I) bromide -100.4 -96.9 107.1 52.4 AgBrO3 Silver(I) bromate -10.5 71.3 151.9 AgCl Silver(I) chloride -127.0 -109.8 96.3 50.8 AgClO3 Silver(I) chlorate -30.3 64.5 142.0 AgClO4 Silver(I) perchlorate -31.1 AgF Silver(I) fluoride -204.6 AgF2 Silver(II) fluoride -360.0 AgI Silver(I) iodide -61.8 -66.2 115.5 56.8 AgIO3 Silver(I) iodate -171.1 -93.7 149.4 102.9 AgNO3 Silver(I) nitrate -124.4 -33.4 140.9 93.1 Ag2 Disilver 410.0 358.8 257.1 37.0 Ag2CrO4 Silver(I) chromate -731.7 -641.8 217.6 142.3 Ag2O Silver(I) oxide -31.1 -11.2 121.3 65.9 Ag2O2 Silver(II) oxide -24.3 27.6 117.0 88.0 Ag2O3 Silver(III) oxide 33.9 121.4 100.0 Ag2O4S Silver(I) sulfate -715.9 -618.4 200.4 131.4 Ag2S Silver(I) sulfide (argentite) -32.6 -40.7 144.0 76.5 Al Aluminum 0.0 330.0 289.4 28.3 164.6 24.4 21.4 AlB3H12 Aluminum borohydride -16.3 13.0 145.0 147.0 289.1 379.2 194.6 AlBr Aluminum monobromide -4.0 -42.0 239.5 35.6 AlBr3 Aluminum tribromide -527.2 -425.1 180.2 100.6 AlCl Aluminum monochloride -47.7 -74.1 228.1 35.0 AlCl2 Aluminum dichloride -331.0 AlCl3 Aluminum trichloride -704.2 -583.2 -628.8 109.3 91.1 AlF Aluminum monofluoride -258.2 -283.7 215.0 31.9 AlF3 Aluminum trifluoride -1510.4 -1204.6 -1431.1 -1188.2 66.5 277.1 75.1 62.6 AlF4Na Sodium tetrafluoroaluminate -

CORINA Classic Program Manual
CORINA Classic Generation of Three-Dimensional Molecular Models Version 4.4.0 Program Description Molecular Networks GmbH September 2021 www.mn-am.com Molecular Networks GmbH Altamira LLC Neumeyerstraße 28 470 W Broad St, Unit #5007 90411 Nuremberg Columbus, OH 43215 Germany USA mn-am.com This document is copyright © 1998-2021 by Molecular Networks GmbH Computerchemie and Altamira LLC. All rights reserved. Except as permitted under the terms of the Software Licensing Agreement of Molecular Networks GmbH Computerchemie, no part of this publication may be reproduced or distributed in any form or by any means or stored in a database retrieval system without the prior written permission of Molecular Networks GmbH or Altamira LLC. The software described in this document is furnished under a license and may be used and copied only in accordance with the terms of such license. (Document version: 4.4.0-2021-09-30) Content Content 1 Introducing CORINA Classic 1 1.1 Objective of CORINA Classic 1 1.2 CORINA Classic in Brief 1 2 Release Notes 3 2.1 CORINA (Full Version) 3 2.2 CORINA_F (Restricted FlexX Interface Version) 25 3 Getting Started with CORINA Classic 26 4 Using CORINA Classic 29 4.1 Synopsis 29 4.2 Options 29 5 Use Cases of CORINA Classic 60 6 Supported File Formats and Interfaces 64 6.1 V2000 Structure Data File (SD) and Reaction Data File (RD) 64 6.2 V3000 Structure Data File (SD) and Reaction Data File (RD) 67 6.3 SMILES Linear Notation 67 6.4 InChI file format 69 6.5 SYBYL File Formats 70 6.6 Brookhaven Protein Data Bank Format (PDB) -

“Solid,” and Stereochemistry Refers to Chemistry in Three Dimensions
CHAPTER 7 STEREOCHEMISTRY he Greek word stereos means “solid,” and stereochemistry refers to chemistry in three dimensions. The foundations of organic stereochemistry were laid by Jacobus Tvan’t Hoff* and Joseph Achille Le Bel in 1874. Independently of each other, van’t Hoff and Le Bel proposed that the four bonds to carbon were directed toward the cor- ners of a tetrahedron. One consequence of a tetrahedral arrangement of bonds to carbon is that two compounds may be different because the arrangement of their atoms in space is different. Isomers that have the same constitution but differ in the spatial arrangement of their atoms are called stereoisomers. We have already had considerable experience with certain types of stereoisomers—those involving cis and trans substitution patterns in alkenes and in cycloalkanes. Our major objectives in this chapter are to develop a feeling for molecules as three- dimensional objects and to become familiar with stereochemical principles, terms, and notation. A full understanding of organic and biological chemistry requires an awareness of the spatial requirements for interactions between molecules; this chapter provides the basis for that understanding. 7.1 MOLECULAR CHIRALITY: ENANTIOMERS Everything has a mirror image, but not all things are superposable on their mirror images. Mirror-image superposability characterizes many objects we use every day. Cups and saucers, forks and spoons, chairs and beds are all identical with their mirror images. Many other objects though—and this is the more interesting case—are not. Your left hand and your right hand, for example, are mirror images of each other but can’t be made to coin- cide point for point, palm to palm, knuckle to knuckle, in three dimensions. -

1 Three-Dimensional Structure of a Molecule
Ch 1 Three dimentional structure of a molecule 1 Three-dimensional structure of a molecule Purpose In this chapter, you will learn that it is sometimes not sufficient to describe the structure of a molecule in a planar, two-dimensional way, which is usually used in textbooks, because the atoms that make up a molecule are arranged three-dimensionally in space. The three-dimensional structures of organic compounds significantly depend on the shapes and characteristics of the hybridized atomic orbitals of the carbon atom. Here you learn about sp3, sp2 and sp hybridized orbitals, which are involved deeply in the structure of molecules. Models are useful tools to conceptualize the shapes of molecules. The commercially available molecular models will be classified based on their features. Sometimes a compound may have two or more names. Similarly, several nomenclatures are used in discussing the stereochemistry of organic compounds. The nomenclature based on the sequence rule will be introduced because this method corresponds to the systematic nomenclature that is most widely used. New terms and concepts molecular formula rational formula structural formula dihedral angle (Compare with tortion angle in Ch. 2) carbon atomic orbital sp3 hybridization tetrahedral structure configurational isomer enantiomer (enantio isomer) Configuration (steric configuration) sp2 hybridization sp hybridization molecular model space filling model skeletal model ball and stick model free rotation restricted rotation sequence rule replica atom Goals of this chapter After you master this chapter successfully, you will be able to do the following: 1to understand that organic compounds are three-dimensional, and that you cannot properly draw these on a two-dimensional sheet of paper. -

Ab Initio Solution of Macromolecular Crystal Structures Without Direct Methods
Ab initio solution of macromolecular crystal structures without direct methods Airlie J. McCoya, Robert D. Oeffnera, Antoni G. Wrobelb, Juha R. M. Ojalac, Karl Tryggvasonc,d, Bernhard Lohkampe, and Randy J. Reada,1 aDepartment of Haematology, Cambridge Institute for Medical Research, University of Cambridge, Cambridge CB2 0XY, United Kingdom; bDepartment of Clinical Biochemistry, Cambridge Institute for Medical Research, University of Cambridge, Cambridge CB2 0XY, United Kingdom; cDivision of Matrix Biology, Department of Medical Biochemistry and Biophysics, Karolinska Institutet, 171 77 Stockholm, Sweden; dCardiovascular and Metabolic Disorders Program, Duke-NUS (National University of Singapore) Medical School, 16957 Singapore; and eDivision of Molecular Structural Biology, Department of Medical Biochemistry and Biophysics, Karolinska Institutet, 171 77 Stockholm, Sweden Edited by Axel T. Brunger, Stanford University, Stanford, CA, and approved February 27, 2017 (received for review January 30, 2017) The majority of macromolecular crystal structures are determined (optionally) a correction for the effect of disordered solvent using the method of molecular replacement, in which known described by the parameters fsol and Bsol: related structures are rotated and translated to provide an initial sffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffi atomic model for the new structure. A theoretical understanding 2 2 Bsol 2π Δ of the signal-to-noise ratio in likelihood-based molecular replace- σA = fP 1 − fsolexp − exp − , [1a] d2 d2 ment searches has been developed to account for the influence of 4 3 model quality and completeness, as well as the resolution of the diffraction data. Here we show that, contrary to current belief, pffiffiffiffi 2π2 Δ2 molecular replacement need not be restricted to the use of models σA ≈ fPexp − .