Captive Behaviour of Cephalopods M.K
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pharaoh Cuttlefish, Sepia Pharaonis, Genome Reveals Unique Reflectin
fmars-08-639670 February 9, 2021 Time: 18:18 # 1 ORIGINAL RESEARCH published: 15 February 2021 doi: 10.3389/fmars.2021.639670 Pharaoh Cuttlefish, Sepia pharaonis, Genome Reveals Unique Reflectin Camouflage Gene Set Weiwei Song1,2, Ronghua Li1,2,3, Yun Zhao1,2, Herve Migaud1,2,3, Chunlin Wang1,2* and Michaël Bekaert3* 1 Key Laboratory of Applied Marine Biotechnology, Ministry of Education, Ningbo University, Ningbo, China, 2 Collaborative Innovation Centre for Zhejiang Marine High-Efficiency and Healthy Aquaculture, Ningbo University, Ningbo, China, 3 Institute of Aquaculture, Faculty of Natural Sciences, University of Stirling, Stirling, United Kingdom Sepia pharaonis, the pharaoh cuttlefish, is a commercially valuable cuttlefish species across the southeast coast of China and an important marine resource for the world fisheries. Research efforts to develop linkage mapping, or marker-assisted selection have been hampered by the absence of a high-quality reference genome. To address this need, we produced a hybrid reference genome of S. pharaonis using a long-read Edited by: platform (Oxford Nanopore Technologies PromethION) to assemble the genome and Andrew Stanley Mount, short-read, high quality technology (Illumina HiSeq X Ten) to correct for sequencing Clemson University, United States errors. The genome was assembled into 5,642 scaffolds with a total length of 4.79 Gb Reviewed by: and a scaffold N of 1.93 Mb. Annotation of the S. pharaonis genome assembly Simo Njabulo Maduna, 50 Norwegian Institute of Bioeconomy identified a total of 51,541 genes, including 12 copies of the reflectin gene, that enable Research (NIBIO), Norway cuttlefish to control their body coloration. -

The Nutritional Values of Ed at Digha Coast, Wes International Journal Of
International Journal of Trend in Scientific Research and Development (IJTSRD) International Open Access Journal ISSN No: 2456 - 6470 | www.ijtsrd.com | Volume - 1 | Issue – 6 Studies the physico-chemical parameters of water, soil and the nutritional values of edible cephalopods found at Digha coast, West Bengal, India Das Manotosh Maity Joydev Research Scholar, Department of Aquaculture Assistant Professor, Department of Aquaculture Management & Technology, Vidyasagar University, Management & Technology, Vidyasagar University, Midnapore, West Bengal, India. Midnapore, West Bengal, India Fishery Field Assistant, Department of Fishery, Government of West Bengal ABSTRACT India is a high speed population growing country and Keywords: Bio-diversity, Cephalopods, Digha Coast, present population of India is about 127 crores. Ecosystem, Molluscs, Nutritional Values Among them a huge number of our children have been suffering from mal-nutritional diseases. They need protein feed and molluscs meat especially INTRODUCTION cephalopods meat is a good source of protein. India harvested 1.73 lakh tones of cephalopods, 0.04 lakh The word ‘Mollusca’ is a Latin word which means tones of bivalves and 0.02 tones of gastropods from ‘soft’. Aristotle is the father of the word ‘Mollusca’. Indian marine resources like Arabian sea, Bay of Molluscs are benthic organisms that live on or in, the Bengal and Indian Ocean in the year 2013-2014. The bottom of the water body with greater than 1.0 mm in people of southern states of India consume molluscs size. Its body is made up of head, visceral mass and meat in huge quantity as their everyday protein locomotory or digging foot, epidermis is forming resource food. -

Taxonomical and Morphometric Studies on Sepia Pharaonis Ehrenberg, 1831(Cephalopoda: Sepioidea) from the Suez Gulf (Red Sea), Egypt
INTERNATIONAL JOURNAL OF ENVIRONMENTAL SCIENCE AND ENGINEERING (IJESE) Vol. 7: 11- 22 (2016) http://www.pvamu.edu/research/activeresearch/researchcenters/texged/ international-journal Prairie View A&M University, Texas, USA Taxonomical and morphometric studies on Sepia pharaonis Ehrenberg, 1831(Cephalopoda: Sepioidea) from the Suez Gulf (Red Sea), Egypt. Rafik Riad1; Manal Atta2; Youssef Halim2 and Noha Elebiary1 1- National Institute of Oceanography and Fisheries, Alexandria branch, Egypt. 2- Faculty of Science, Alexandria University, Egypt. ARTICLE INFO ABSTRACT Article History Morphometric characters of male and female Sepia pharaonis Received: Feb.2016 were investigated for samples obtained from commercial trawling Accepted: April, 2016 vessels of Suez Gulf, Egypt. Samples were collected (850 Available online: Jan. 2017 individuals) between winter 2014 to autumn 2014.Measurements for _________________ the smallest and largest male and female specimens, mean and Keywords: Taxonomy number of parts showed negative allometric growth (slope less than Morphology 1). Generally, the coefficient of determination R for MW, HL, HW, Sepia pharaonis FL, FW, FU.L, FU.W, AL and TL (0.9766, 0.9551, 0.9767, 0.9965, Suez Gulf 0.9453, 0.9779, 0.9712, 0.9580, 0.9685), respectively, were high for Red Sea most measurements. Egypt The present study reported some additional characters for this species that were not recorded before from other previous descriptions 1. INTRODUCTION Cephalopods are characterized by their activity, intelligent carnivorous creatures with highly advanced visual and nervous system (Boyle and Rodhouse, 2005) .They are soft- bodied bilaterally symmetrical animals with a well-developed head and body that consists of the muscular undivided mantle, mantle cavity houses the internal organs and also houses the external fins when present. -

Is Sepiella Inermis ‘Spineless’?
IOSR Journal of Pharmacy and Biological Sciences (IOSR-JPBS) e-ISSN:2278-3008, p-ISSN:2319-7676. Volume 12, Issue 5 Ver. IV (Sep. – Oct. 2017), PP 51-60 www.iosrjournals.org Is Sepiella inermis ‘Spineless’? 1 Visweswaran B * 1Department of Zoology, K.M. Centre for PG Studies (Autonomous), Lawspet Campus, Pondicherry University, Puducherry-605 008, India. *Corresponding Author: Visweswaran B Abstract: Many a report seemed to project at a noble notion of having identified some novel and bioactive compounds claimed to have been found from Sepiella inermis; but lagged to log their novelty scarcely defined due to certain technical blunders they seem to have coldly committed in such valuable pieces of aboriginal research works, reported to have sophistically been accomplished but unnoticed with considerable lack of significant finesse. They have dealt with finer biochemicals already been reported to have been available from S.inermis; yet, to one’s dismay, have failed to maintain certain conventional means meant for original research. This quality review discusses about the illogical math rooting toward and logical aftermath branching from especially certain spectral reports. Keywords: Sepiella inermis, ink, melanin, DOPA ----------------------------------------------------------------------------------------------------------------------------- ---------- Date of Submission: 16-09-2017 Date of acceptance: 28-09-2017 ----------------------------------------------------------------------------------------------------------------------------- ---------- I. Introduction Sepiella inermis is a demersally 1 bentho-nektonic 2, Molluscan, cephalopod ‗spineless‘ cuttlefish species, with invaluable juveniles 3, from the megametrical Indian coast 4-6, as incidental catches in shore seine 7 & 8, as egg clusters 9 from shallow waters 1 after monsoon at Vizhinjam coast 10 and Goa coast 11 of India and sundried, abundantly but rarely 8. II. -

Surat Thani Blue Swimming Crab Fishery Improvement Project
Surat Thani Blue Swimming Crab Fishery Improvement Project -------------------------------------------------------------------------------------------------------------------------------------- Milestone 33b: Final report of bycatch research Progress report: The study of fishery biology, socio-economic and ecosystem related to the restoration of Blue Swimming Crab following Fishery improvement program (FIP) in Bandon Bay, Surat Thani province. Amornsak Sawusdee1 (1) The Center of Academic Service, Walailak University, Tha Sala, Nakhon Si Thammarat, 80160 The results of observation of catching BSC by using collapsible crab trap and floating seine. According to the observation of aquatic animal which has been caught by main BSC fishing gears; floating seine and collapsible crab trap, there were 176 kind of aquatic animals. The catch aquatic animals are shown in the table1. In this study, aquatic animal was classified into 11 Groups; Blue Swimming Crab (Portunus Pelagicus), Coelenterata (coral animals, true jellies, sea anemones, sea pens), Helcionelloida (clam, bivalve, gastropod), Cephalopoda (sqiud, octopus), Chelicerata (horseshoe crab), Hoplocari(stomatopods), Decapod (shrimp), Anomura (hermit crab), Brachyura (crab), Echinoderm (sea cucambers, sea stars, sea urchins), Vertebrata (fish). Vertebrata was the main group that was captured by BSC fishing gears, more than 70 species. Next are Helcionelloida and Helcionelloida 38 species and 29 species respectively. The sample that has been classified were photographed and attached in appendix 1. However, some species were classified as unknow which are under the classification process and reconcile. There were 89 species that were captured by floating seine. The 3 main group that were captured by this fishing gear are Vertebrata (34 species), Brachyura (20 species) Helcionelloida and Echinoderm (10 Species). On the other hand, there were 129 species that were captured by collapsible crab trap. -

Os Nomes Galegos Dos Moluscos 2020 2ª Ed
Os nomes galegos dos moluscos 2020 2ª ed. Citación recomendada / Recommended citation: A Chave (20202): Os nomes galegos dos moluscos. Xinzo de Limia (Ourense): A Chave. https://www.achave.ga /wp!content/up oads/achave_osnomesga egosdos"mo uscos"2020.pd# Fotografía: caramuxos riscados (Phorcus lineatus ). Autor: David Vilasís. $sta o%ra est& su'eita a unha licenza Creative Commons de uso a%erto( con reco)ecemento da autor*a e sen o%ra derivada nin usos comerciais. +esumo da licenza: https://creativecommons.org/ icences/%,!nc-nd/-.0/deed.g . Licenza comp eta: https://creativecommons.org/ icences/%,!nc-nd/-.0/ ega code. anguages. 1 Notas introdutorias O que cont!n este documento Neste recurso léxico fornécense denominacións para as especies de moluscos galegos (e) ou europeos, e tamén para algunhas das especies exóticas máis coñecidas (xeralmente no ámbito divulgativo, por causa do seu interese científico ou económico, ou por seren moi comúns noutras áreas xeográficas) ! primeira edición d" Os nomes galegos dos moluscos é do ano #$%& Na segunda edición (2$#$), adicionáronse algunhas especies, asignáronse con maior precisión algunhas das denominacións vernáculas galegas, corrixiuse algunha gralla, rema'uetouse o documento e incorporouse o logo da (have. )n total, achéganse nomes galegos para *$+ especies de moluscos A estrutura )n primeiro lugar preséntase unha clasificación taxonómica 'ue considera as clases, ordes, superfamilias e familias de moluscos !'uí apúntanse, de maneira xeral, os nomes dos moluscos 'ue hai en cada familia ! seguir -

Population Dynamics of the Pharaoh Cuttlefish Sepia Pharaonis (Mollusca: Cephalopoda) in the Arabian Sea Coast of Oman
Indian J. Fish., 61(1) : 7-11, 2014 Population dynamics of the pharaoh cuttlefish Sepia pharaonis (Mollusca: Cephalopoda) in the Arabian Sea coast of Oman SAHAR FAHMY MEHANNA, LUBNA AL-KHARUSI*AND SAOUD AL-HABSI* National Institute of Oceanography and Fisheries, P.O. Box 182, Egypt *Marine Science and Fisheries Centre, P. O. Box 427, P. C. 100, Muscat, Oman e-mail : [email protected] ABSTRACT The stock of pharaoh cuttlefish Sepia pharaonis was assessed based on 4616 specimens (1895 males, 2051 females and 670 unsexed), collected during the demersal fishery survey in the Arabian Sea coast of Oman between September 2007 and August 2008 and from November 2011 to June 2012. Age and growth were studied based on length frequency data using Bhattacharya’s method. There was no significant difference in growth between sexes and the longevity was estimated to be five years for mantle length (ML) range 1.7 – 44 cm. The estimated von Bertalanffy growth parameters were ∞L = 46.21 cm ML, -1 K = 0.52 y and t0 = 0.1 y. The instantaneous rates of total (Z), natural (M) and fishing (F) mortalities were 2.57, 0.997 and 1.57 y-1 respectively with exploitation rate (E) of 0.61 and exploitation ratio (U) of 0.66. The estimated ML at first capture (Lc) was 12 cm, while the ML at first sexual maturity (Lm) was 19.60 cm. The yield per recruit model revealed that S. pharaonis stock in the Arabian Sea is heavily exploited, but has a scope to increase the yield by increasing the length at first capture to be not less than 20 cm ML. -

Bioaccumulation of Heavy Metals in Cuttlefish Sepiella Inermis from Visakhapatnam Coastal Waters
International Journal of Science and Research (IJSR) ISSN: 2319-7064 Index Copernicus Value (2016): 79.57 | Impact Factor (2017): 7.296 Bioaccumulation of Heavy Metals in Cuttlefish Sepiella Inermis from Visakhapatnam Coastal Waters R.Rekha1, B. Ganga Rao2 1Department of Foods, Nutrition & Dietetics, Andhra University, Visakhapatnam-530 003, India 2Professor, Department of Pharmaceutical Sciences, Andhra University, Visakhapatnam-530 003, India Abstract: This study investigates 9 elements both essential (Cr, Cu, Zn, Fe, Mn and Ni) and non essential (Cd, Hg and Pb) in the tissues and whole of the cuttlefish Sepiella inermis caught off Visakhapatnam coast (east coast of India, Bay of Bengal). The level of elements was determined by atomic absorption spectrophotometry (AAS). The concentration ranges found for these heavy metals, expressed on a wet weight basis, were as follows: Hg, Cd, Pb, Cu, Zn, Fe, Mn, Cr, and Ni concentrations in cuttlefish muscle samples were 0.01 - 0.07, 0.11-0.67, 0.11-1.14, 0.52-6.08, 4.82-19.32, 0.08-5.84, 0-0.49 and 0-2.11, 0-0.92 ppm respectively. As for other cephalopod species, the liver showed the highest concentrations of many elements highlighting their role in bioaccumulation and detoxification processes. The mean values of highly hazardous metals in the muscle of the Sepiella inermis, were: Hg = 0.04, Cd = 0.481, Pb = 0.525, Cr = 0.662, all within the international safety limits. The level of contamination in Sepiella inermis by these heavy metals is compared to those studied in other parts of the world and the legal standards set by international legalizations. -

ASFIS ISSCAAP Fish List February 2007 Sorted on Scientific Name
ASFIS ISSCAAP Fish List Sorted on Scientific Name February 2007 Scientific name English Name French name Spanish Name Code Abalistes stellaris (Bloch & Schneider 1801) Starry triggerfish AJS Abbottina rivularis (Basilewsky 1855) Chinese false gudgeon ABB Ablabys binotatus (Peters 1855) Redskinfish ABW Ablennes hians (Valenciennes 1846) Flat needlefish Orphie plate Agujón sable BAF Aborichthys elongatus Hora 1921 ABE Abralia andamanika Goodrich 1898 BLK Abralia veranyi (Rüppell 1844) Verany's enope squid Encornet de Verany Enoploluria de Verany BLJ Abraliopsis pfefferi (Verany 1837) Pfeffer's enope squid Encornet de Pfeffer Enoploluria de Pfeffer BJF Abramis brama (Linnaeus 1758) Freshwater bream Brème d'eau douce Brema común FBM Abramis spp Freshwater breams nei Brèmes d'eau douce nca Bremas nep FBR Abramites eques (Steindachner 1878) ABQ Abudefduf luridus (Cuvier 1830) Canary damsel AUU Abudefduf saxatilis (Linnaeus 1758) Sergeant-major ABU Abyssobrotula galatheae Nielsen 1977 OAG Abyssocottus elochini Taliev 1955 AEZ Abythites lepidogenys (Smith & Radcliffe 1913) AHD Acanella spp Branched bamboo coral KQL Acanthacaris caeca (A. Milne Edwards 1881) Atlantic deep-sea lobster Langoustine arganelle Cigala de fondo NTK Acanthacaris tenuimana Bate 1888 Prickly deep-sea lobster Langoustine spinuleuse Cigala raspa NHI Acanthalburnus microlepis (De Filippi 1861) Blackbrow bleak AHL Acanthaphritis barbata (Okamura & Kishida 1963) NHT Acantharchus pomotis (Baird 1855) Mud sunfish AKP Acanthaxius caespitosa (Squires 1979) Deepwater mud lobster Langouste -

DNA Barcoding Reveal Patterns of Species Diversity Among
www.nature.com/scientificreports OPEN DNA barcoding reveal patterns of species diversity among northwestern Pacific molluscs Received: 04 April 2016 Shao’e Sun, Qi Li, Lingfeng Kong, Hong Yu, Xiaodong Zheng, Ruihai Yu, Lina Dai, Yan Sun, Accepted: 25 August 2016 Jun Chen, Jun Liu, Lehai Ni, Yanwei Feng, Zhenzhen Yu, Shanmei Zou & Jiping Lin Published: 19 September 2016 This study represents the first comprehensive molecular assessment of northwestern Pacific molluscs. In total, 2801 DNA barcodes belonging to 569 species from China, Japan and Korea were analyzed. An overlap between intra- and interspecific genetic distances was present in 71 species. We tested the efficacy of this library by simulating a sequence-based specimen identification scenario using Best Match (BM), Best Close Match (BCM) and All Species Barcode (ASB) criteria with three threshold values. BM approach returned 89.15% true identifications (95.27% when excluding singletons). The highest success rate of congruent identifications was obtained with BCM at 0.053 threshold. The analysis of our barcode library together with public data resulted in 582 Barcode Index Numbers (BINs), 72.2% of which was found to be concordantly with morphology-based identifications. The discrepancies were divided in two groups: sequences from different species clustered in a single BIN and conspecific sequences divided in one more BINs. In Neighbour-Joining phenogram, 2,320 (83.0%) queries fromed 355 (62.4%) species-specific barcode clusters allowing their successful identification. 33 species showed paraphyletic and haplotype sharing. 62 cases are represented by deeply diverged lineages. This study suggest an increased species diversity in this region, highlighting taxonomic revision and conservation strategy for the cryptic complexes. -

POPULATION DYNAMICS of the HOODED CUTTLEFISH Sepia Prashadi (WINCKWORTH, 1936) from the OMANI COASTAL WATERS of the ARABIAN SEA
7(1): 89-98 (2013) DOI: 10.3153/jfscom.2013010 Journal of FisheriesSciences.com E-ISSN 1307-234X © 2013 www.fisheriessciences.com RESEARCH ARTICLE ARAŞTIRMA MAKALESİ POPULATION DYNAMICS OF THE HOODED CUTTLEFISH Sepia prashadi (WINCKWORTH, 1936) FROM THE OMANI COASTAL WATERS OF THE ARABIAN SEA Sahar F. Mehanna∗, Dawood Al-Mamry Marine Science and Fisheries Centre, Muscat, OMAN Abstract: Basic population parameters of the hooded cuttlefish Sepia prashadi, in the Arabian Sea were described from samples collected during the demersal trawl survey of the Arabian Sea between September 2007 and August 2008. A total of 6869 S. prashadi with mantle lengths (ML) ranged from 3.4 to 21.2 cm were analyzed. Age and growth were studied using progression analysis model by applying Bhattacharya method. There were no significant differences in population parameters between sexes. The asymptotic ML was 24.13 cm, while the growth co- efficient K was 0.81/year and t0= -0.14 year. Mean total, natural and fishing mortalities were 3.66, 1.54 and 2.12 per year respectively. The exploitation ratio (E =0.58) suggests that the fishing pressure on S. prashadi in the Omani coastal waters is slightly high. Relative yield per recruit and relative biomass per recruit analysis showed that S. prashadi stock in the Arabian Sea is in its optimum situation as the current E is lower than that which gives the maximum Y’/R. For the management purpose and to reduce the risk due to the sampling bias, the current exploitation rate should be reduced by about 38% to achieve E0.5 as a target reference point and the present length at first capture should be raised to about 14 cm ML to conserve the first spawners of the stock. -
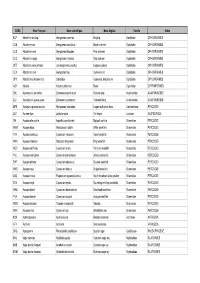
Nom Français
CODE Nom Français Nom scientifique Nom Anglais Famille Ordre KCP Abadèche du Cap Genypterus capensis Kingklip Ophidiidae OPHIDIIFORMES CUB Abadèche noir Genypterus maculatus Black cusk-eel Ophidiidae OPHIDIIFORMES CUS Abadèche rosé Genypterus blacodes Pink cusk-eel Ophidiidae OPHIDIIFORMES CUC Abadèche rouge Genypterus chilensis Red cusk-eel Ophidiidae OPHIDIIFORMES OFZ Abadèche sans jambes Lamprogrammus exutus Legless cuskeel Ophidiidae OPHIDIIFORMES CEX Abadèches nca Genypterus spp Cusk-eels nei Ophidiidae OPHIDIIFORMES OPH Abadèches, brotules nca Ophidiidae Cusk-eels, brotulas nei Ophidiidae OPHIDIIFORMES ALR Ablette Alburnus alburnus Bleak Cyprinidae CYPRINIFORMES ZML Acanthure à pierreries Zebrasoma gemmatum Spotted tang Acanthuridae ACANTHUROIDEI ZLV Acanthure à queue jaune Zebrasoma xanthurum Yellowtail tang Acanthuridae ACANTHUROIDEI MPS Achigan à grande bouche Micropterus salmoides Largemouth black bass Centrarchidae PERCOIDEI LQT Acmée râpe Lottia limatula File limpet Lottiidae GASTROPODA ISA Acoupa aile-courte Isopisthus parvipinnis Bigtooth corvina Sciaenidae PERCOIDEI WEW Acoupa blanc Atractoscion nobilis White weakfish Sciaenidae PERCOIDEI YNV Acoupa cambucu Cynoscion virescens Green weakfish Sciaenidae PERCOIDEI WKK Acoupa chasseur Macrodon ancylodon King weakfish Sciaenidae PERCOIDEI WEP Acoupa du Pérou Cynoscion analis Peruvian weakfish Sciaenidae PERCOIDEI YNJ Acoupa mongolare Cynoscion jamaicensis Jamaica weakfish Sciaenidae PERCOIDEI SWF Acoupa pintade Cynoscion nebulosus Spotted weakfish Sciaenidae PERCOIDEI WKS Acoupa