Stink Bugs: Spatial Distribution, Pecan Phenological Susceptibility and Sampling Program Brian Cowell University of Arkansas, Fayetteville
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sunday, March 4, 2012
Joint Meeting of the Southeastern and Southwestern Branches Entomological Society of America 4-7 March 2012 Little Rock, Arkansas 0 Dr. Norman C. Leppla President, Southeastern Branch of the Entomological Society of America, 2011-2012 Dr. Allen E. Knutson President, Southwestern Branch of the Entomological Society of America, 2011-2012 1 2 TABLE OF CONTENTS Presidents Norman C. Leppla (SEB) and Allen E. 1 Knutson (SWB) ESA Section Names and Acronyms 5 PROGRAM SUMMARY 6 Meeting Notices and Policies 11 SEB Officers and Committees: 2011-2012 14 SWB Officers and Committees: 2011-2012 16 SEB Award Recipients 19 SWB Award Recipients 36 SCIENTIFIC PROGRAM SATURDAY AND SUNDAY SUMMARY 44 MONDAY SUMMARY 45 Plenary Session 47 BS Student Oral Competition 48 MS Student Oral Competition I 49 MS Student Oral Competition II 50 MS Student Oral Competition III 52 MS Student Oral Competition IV 53 PhD Student Oral Competition I 54 PhD Student Oral Competition II 56 BS Student Poster Competition 57 MS Student Poster Competition 59 PhD Student Poster Competition 62 Linnaean Games Finals/Student Awards 64 TUESDAY SUMMARY 65 Contributed Papers: P-IE (Soybeans and Stink Bugs) 67 Symposium: Spotted Wing Drosophila in the Southeast 68 Armyworm Symposium 69 Symposium: Functional Genomics of Tick-Pathogen 70 Interface Contributed Papers: PBT and SEB Sections 71 Contributed Papers: P-IE (Cotton and Corn) 72 Turf and Ornamentals Symposium 73 Joint Awards Ceremony, Luncheon, and Photo Salon 74 Contributed Papers: MUVE Section 75 3 Symposium: Biological Control Success -

He Great Lakes Entomologist
The Great Lakes Entomologist Volume 26 Number 4 - Winter 1994 Number 4 - Winter Article 2 1994 December 1994 Comparative Study of Life Histories, Laboratory Rearing, and Immature Stages of Euschistus Servus and Euschistus Variolarius (Hemiptera: Pentatomidae) Joseph Munyaneza Southern Illinois University J. E. McPherson Southern Illinois University Follow this and additional works at: https://scholar.valpo.edu/tgle Part of the Entomology Commons Recommended Citation Munyaneza, Joseph and McPherson, J. E. 1994. "Comparative Study of Life Histories, Laboratory Rearing, and Immature Stages of Euschistus Servus and Euschistus Variolarius (Hemiptera: Pentatomidae)," The Great Lakes Entomologist, vol 26 (4) Available at: https://scholar.valpo.edu/tgle/vol26/iss4/2 This Peer-Review Article is brought to you for free and open access by the Department of Biology at ValpoScholar. It has been accepted for inclusion in The Great Lakes Entomologist by an authorized administrator of ValpoScholar. For more information, please contact a ValpoScholar staff member at [email protected]. Munyaneza and McPherson: Comparative Study of Life Histories, Laboratory Rearing, and Imma 1994 THE GREAT LAKES ENTOMOLOGIST 263 COMPARATIVE STUDY OF LIFE HISTORIES, LABORATORY REARING, AND IMMATURE STAGES OF EUSCHISTUS SERVUS AND EUSCHISTUS VARIOLARIUS (HEMIPTERA:PENTATOMIDAE)l Joseph Munyaneza and J. E. McPherson2 ABSTRACT A comparative study was conducted of the field life histories of Euschis tus servus and E. varialarius in southern Illinois, their life cycles under con trolled laboratory conditions, and their immature stages. The results indicate that E. servus is bivoltine and E. variolarius is univol tine. Adults of both species emerged from overwintering sites during early April, began feeding and copulating on leaves of common mullein (Verbascum thapsus) and surrounding vegetation, and reproduced shortly thereafter. -
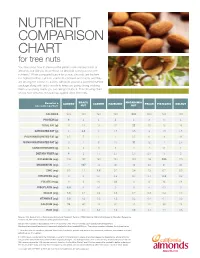
Nutrient Comparison Chart
NUTRIENT COMPARISON CHART for tree nuts You may know how to measure the perfect one-ounce portion of almonds, but did you know those 23 almonds come packed with nutrients? When compared ounce for ounce, almonds are the tree nut highest in fiber, calcium, vitamin E, riboflavin and niacin, and they are among the lowest in calories. Almonds provide a powerful nutrient package along with tasty crunch to keep you going strong, making them a satisfying snack you can feel good about. The following chart shows how almonds measure up against other tree nuts. BRAZIL MACADAMIA Based on a ALMOND CASHEW HAZELNUT PECAN PISTACHIO WALNUT one-ounce portion1 NUT NUT CALORIES 1602 190 160 180 200 200 160 190 PROTEIN (g) 6 4 4 4 2 3 6 4 TOTAL FAT (g) 14 19 13 17 22 20 13 19 SATURATED FAT (g) 1 4.5 3 1.5 3.5 2 1.5 1.5 POLYUNSATURATED FAT (g) 3.5 7 2 2 0.5 6 4 13 MONOUNSATURATED FAT (g) 9 7 8 13 17 12 7 2.5 CARBOHYDRATES (g) 6 3 9 5 4 4 8 4 DIETARY FIBER (g) 4 2 1.5 2.5 2.5 2.5 3 2 POTASSIUM (mg) 208 187 160 193 103 116 285 125 MAGNESIUM (mg) 77 107 74 46 33 34 31 45 ZINC (mg) 0.9 1.2 1.6 0.7 0.4 1.3 0.7 0.9 VITAMIN B6 (mg) 0 0 0.1 0.2 0.1 0.1 0.3 0.2 FOLATE (mcg) 12 6 20 32 3 6 14 28 RIBOFLAVIN (mg) 0.3 0 0.1 0 0 0 0.1 0 NIACIN (mg) 1.0 0.1 0.4 0.5 0.7 0.3 0.4 0.3 VITAMIN E (mg) 7.3 1.6 0.3 4.3 0.2 0.4 0.7 0.2 CALCIUM (mg) 76 45 13 32 20 20 30 28 IRON (mg) 1.1 0.7 1.7 1.3 0.8 0.7 1.1 0.8 Source: U.S. -

The Pentatomidae, Or Stink Bugs, of Kansas with a Key to Species (Hemiptera: Heteroptera) Richard J
Fort Hays State University FHSU Scholars Repository Biology Faculty Papers Biology 2012 The eP ntatomidae, or Stink Bugs, of Kansas with a key to species (Hemiptera: Heteroptera) Richard J. Packauskas Fort Hays State University, [email protected] Follow this and additional works at: http://scholars.fhsu.edu/biology_facpubs Part of the Biology Commons, and the Entomology Commons Recommended Citation Packauskas, Richard J., "The eP ntatomidae, or Stink Bugs, of Kansas with a key to species (Hemiptera: Heteroptera)" (2012). Biology Faculty Papers. 2. http://scholars.fhsu.edu/biology_facpubs/2 This Article is brought to you for free and open access by the Biology at FHSU Scholars Repository. It has been accepted for inclusion in Biology Faculty Papers by an authorized administrator of FHSU Scholars Repository. 210 THE GREAT LAKES ENTOMOLOGIST Vol. 45, Nos. 3 - 4 The Pentatomidae, or Stink Bugs, of Kansas with a key to species (Hemiptera: Heteroptera) Richard J. Packauskas1 Abstract Forty eight species of Pentatomidae are listed as occurring in the state of Kansas, nine of these are new state records. A key to all species known from the state of Kansas is given, along with some notes on new state records. ____________________ The family Pentatomidae, comprised of mainly phytophagous and a few predaceous species, is one of the largest families of Heteroptera. Some of the phytophagous species have a wide host range and this ability may make them the most economically important family among the Heteroptera (Panizzi et al. 2000). As a group, they have been found feeding on cotton, nuts, fruits, veg- etables, legumes, and grain crops (McPherson 1982, McPherson and McPherson 2000, Panizzi et al 2000). -

A Stink Bug Euschistus Quadrator Rolston (Insecta: Hemiptera: Pentatomidae)1 Sara A
EENY-523 A Stink Bug Euschistus quadrator Rolston (Insecta: Hemiptera: Pentatomidae)1 Sara A. Brennan, Joseph Eger, and Oscar E. Liburd2 Introduction in the membranous area of the hemelytra, a characteristic present in other Euschistus species. Euschistus quadrator Rolston was described in 1974, with specimens from Mexico, Texas, and Louisiana. Euschistus quadrator was not found in Florida until 1992. It has since spread throughout the state as well as becoming an agricultural pest of many fruit, vegetable, and nut crops in the southeastern United States. It has a wide host range, but is most commonly found in cotton, soybean and corn. Euschistus quadrator has recently become a more promi- nent pest with the introduction of crops such as Bt cotton and an increase in the usage of biorational or reduced-risk pesticides. Distribution Euschistus quadrator is originally from Texas and Mexico, and has since been reported in Louisiana, Georgia, and Florida. Description Figure 1. Dorsal view of Euschistus quadrator Rolston; adult male (left) Adults and female (right), a stink bug. Credits: Lyle Buss, University of Florida The adults are shield-shaped and light to dark brown in color. They are smaller than many other members of the ge- Eggs nus, generally less than 11 mm in length and approximately Euschistus quadrator eggs are initially semi-translucent and 5 mm wide across the abdomen. They are similar in size to light yellow, and change color to red as the eggs mature. The Euschistus obscurus. Euschistus quadrator lacks dark spots micropylar processes (fan-like projections around the top 1. This document is EENY-523, one of a series of the Department of Entomology and Nematology, UF/IFAS Extension. -
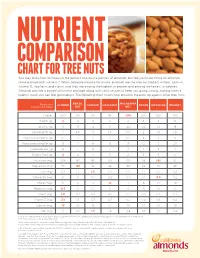
Chart for Tree Nuts
NUTRIENT COMPARISON CHART FOR TREE NUTS You may know how to measure the perfect one-ounce portion of almonds, but did you know those 23 almonds come packed with nutrients? When compared ounce for ounce, almonds are the tree nut highest in fiber, calcium, vitamin E, riboflavin and niacin, and they are among the highest in protein and among the lowest in calories. Almonds provide a powerful nutrient package along with tasty crunch to keep you going strong, making them a healthy snack you can feel good about. The following chart shows how almonds measure up against other tree nuts. BRAZIL MACADAMIA Based on a ALMOND CASHEW HAZELNUT PECAN PISTACHIO WALNUT one-ounce portion1 NUT NUT Calories 1602 190 160 180 200 200 160 190 Protein (g) 6 4 4 4 2 3 6 4 Total Fat (g) 14 19 13 17 22 20 13 18 Saturated Fat (g) 1 4.5 3 1.5 3.5 2 1.5 1.5 Polyunsaturated Fat (g) 3.5 7 2 2 0.5 6 4 13 Monounsaturated Fat (g) 9 7 8 13 17 12 7 2.5 Carbohydrates (g) 6 3 9 5 4 4 8 4 Dietary Fiber (g) 4 2 1 3 2 3 3 2 Potassium (mg) 208 187 160 193 103 116 285 125 Magnesium (mg) 77 107 74 46 33 34 31 45 Zinc (mg) 0.9 1.2 1.6 0.7 0.4 1.3 0.7 0.9 Vitamin B6 (mg) 0 0 0.1 0.2 0.1 0.1 0.3 0.2 Folate (mcg) 12 6 20 32 3 6 14 28 Riboflavin (mg) 0.3 0 0.1 0 0 0 0.1 0 Niacin (mg) 1.0 0.1 0.4 0.5 0.7 0.3 0.4 0.3 Vitamin E (mg) 7.3 1.6 0.3 4.3 0.2 0.4 0.6 0.2 Calcium (mg) 76 45 13 32 20 20 30 28 Iron (mg) 1.1 0.7 1.7 1.3 0.8 0.7 1.1 0.8 Source: U.S. -

WO 2017/023486 Al 9 February 2017 (09.02.2017) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2017/023486 Al 9 February 2017 (09.02.2017) P O P C T (51) International Patent Classification: 0552 (US). FENGLER, Kevin; 7250 NW 62nd Ave, P.O. AOlH l/00 (2006.01) C07K 14/195 (2006.01) Box 552, Johnston, IA 5013 1-0552 (US). SCHEPERS, A01H3/00 (2006.01) C12N 15/82 (2006.01) Eric; 7250 NW 62nd Ave, P.O. Box 552, Johnston, IA 5013 1-0552 (US). UDRANSZKY, Ingrid; 7250 NW 62nd (21) International Application Number: Ave, P.O. Box 552, Johnston, IA 5013 1-0552 (US). PCT/US20 16/04 1452 (74) Agent: BAUER, S., Christopher; Pioneer Hi-Bred Inter (22) International Filing Date: national, Inc., 7100 N.W. 62nd Avenue, Johnston, IA 8 July 2016 (08.07.2016) 5013 1-1014 (US). (25) Filing Language: English (81) Designated States (unless otherwise indicated, for every (26) Publication Language: English kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, (30) Priority Data: BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, 62/201,977 6 August 2015 (06.08.2015) US DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (71) Applicants: PIONEER HI-BRED INTERNATIONAL, HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, INC. [US/US]; PIONEER HI-BRED INTERNATIONAL, KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, INC., 7100 N.W. -

American Pecan
THE AMERICAN PECAN CRACKING OPEN THE NUTRITION STORY OF OUR NATIVE NUT Scientific evidence suggests but does not prove YOU THINK OF THEM FOR PIE. that eating 1.5 ounces per day of most nuts, YOU ADORE THEM IN PRALINES. such as pecans, as part of a diet low in saturated BUT DID YOU KNOW PECANS fat and cholesterol may reduce the risk of ARE ACTUALLY A NUTRITION heart disease.* POWERHOUSE? U.S. Food and Drug Administration Don’t be fooled by their rich, buttery texture and naturally sweet taste, pecans are extremely *One serving of pecans (28g) contains 18g of unsaturated fat and only 2g of saturated fat. nutrient dense. The macronutrient profile of pecans is appealing to many people: Pecans contain the same beneficial unsaturated protein (3 grams), carbohydrate (4 grams) and fat (20 grams). fats that are found in other nuts, and nearly two decades of research suggests that nuts, including A handful of pecans – about 19 halves – is a good source of fiber, pecans, may help promote heart health. thiamin, and zinc, and an excellent source of copper and manganese – a mineral that’s essential for metabolism and In each 1-ounce serving of raw pecans you’ll get bone health. 12 grams of “good” monounsaturated fat, with zero cholesterol or sodium.1 Compared to other To top it off, pecans contain polyphenols, specifically flavonoids nuts, pecans are among the lowest in carbs and – which are the types of bioactive compounds found in brightly highest in fiber. colored produce.2 So think of pecans as a supernut. -

Juglans Nigra Juglandaceae L
Juglans nigra L. Juglandaceae LOCAL NAMES English (walnut,American walnut,eastern black walnut,black walnut); French (noyer noir); German (schwarze Walnuß); Portuguese (nogueira- preta); Spanish (nogal negro,nogal Americano) BOTANIC DESCRIPTION Black walnut is a deciduous tree that grows to a height of 46 m but ordinarily grows to around 25 m and up to 102 cm dbh. Black walnut develops a long, smooth trunk and a small rounded crown. In the open, the trunk forks low with a few ascending and spreading coarse branches. (Robert H. Mohlenbrock. USDA NRCS. The root system usually consists of a deep taproot and several wide- 1995. Northeast wetland flora: Field office spreading lateral roots. guide to plant species) Leaves alternate, pinnately compound, 30-70 cm long, up to 23 leaflets, leaflets are up to 13 cm long, serrated, dark green with a yellow fall colour in autumn and emits a pleasant sweet though resinous smell when crushed or bruised. Flowers monoecious, male flowers catkins, small scaley, cone-like buds; female flowers up to 8-flowered spikes. Fruit a drupe-like nut surrounded by a fleshy, indehiscent exocarp. The nut has a rough, furrowed, hard shell that protects the edible seed. Fruits Bark (Robert H. Mohlenbrock. USDA NRCS. 1995. Northeast wetland flora: Field office produced in clusters of 2-3 and borne on the terminals of the current guide to plant species) season's growth. The seed is sweet, oily and high in protein. The bitter tasting bark on young trees is dark and scaly becoming darker with rounded intersecting ridges on maturity. BIOLOGY Flowers begin to appear mid-April in the south and progressively later until early June in the northern part of the natural range. -

Assessment of Stink Bug Feeding Damage in Louisiana Soybean
Louisiana State University LSU Digital Commons LSU Master's Theses Graduate School 2012 Assessment of stink bug feeding damage in Louisiana soybean: use of a no-choice feeding field protocol Jessica Leigh Parker Louisiana State University and Agricultural and Mechanical College, [email protected] Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_theses Part of the Entomology Commons Recommended Citation Parker, Jessica Leigh, "Assessment of stink bug feeding damage in Louisiana soybean: use of a no-choice feeding field protocol" (2012). LSU Master's Theses. 809. https://digitalcommons.lsu.edu/gradschool_theses/809 This Thesis is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Master's Theses by an authorized graduate school editor of LSU Digital Commons. For more information, please contact [email protected]. ASSESSMENT OF STINK BUG FEEDING DAMAGE IN LOUISIANA SOYBEAN: USE OF A NO-CHOICE FEEDING FIELD PROTOCOL A Thesis Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requirements for the degree of Master of Science in The Department of Entomology by Jessica Leigh Parker B.S. Texas A&M University, 2008 May, 2012 ACKNOWLEDGEMENTS I wish to express my appreciation to the Louisiana State University Agricultural Center, the faculty and staff of the Louisiana State University Department of Entomology, and the personnel at the Macon Ridge Research Station. Thanks to the numerous student workers of the Macon Ridge Research Station for many hours of hard work collecting stink bugs and executing infestations in the humid Louisiana heat, without their help completion of this project would have been nearly impossible. -

Hemiptera: Heteroptera: Pentatomoidea
VIVIANA CAUDURO MATESCO SISTEMÁTICA DE THYREOCORIDAE AMYOT & SERVILLE (HEMIPTERA: HETEROPTERA: PENTATOMOIDEA): REVISÃO DE ALKINDUS DISTANT, MORFOLOGIA DO OVO DE DUAS ESPÉCIES DE GALGUPHA AMYOT & SERVILLE E ANÁLISE CLADÍSTICA DE CORIMELAENA WHITE, COM CONSIDERAÇÕES SOBRE A FILOGENIA DE THYREOCORIDAE, E MORFOLOGIA DO OVO DE 16 ESPÉCIES DE PENTATOMIDAE COMO EXEMPLO DO USO DE CARACTERES DE IMATUROS EM FILOGENIAS Tese apresentada ao Programa de Pós-Graduação em Biologia Animal, Instituto de Biociências, Universidade Federal do Rio Grande do Sul, como requisito parcial à obtenção do Título de Doutor em Biologia Animal. Área de concentração: Biologia Comparada Orientadora: Profa. Dra. Jocelia Grazia Co-Orientador: Prof. Dr. Cristiano F. Schwertner UNIVERSIDADE FEDERAL DO RIO GRANDE DO SUL PORTO ALEGRE 2014 “Sistemática de Thyreocoridae Amyot & Serville (Hemiptera: Heteroptera: Pentatomoidea): revisão de Alkindus Distant, morfologia do ovo de duas espécies de Galgupha Amyot & Serville e análise cladística de Corimelaena White, com considerações sobre a filogenia de Thyreocoridae, e morfologia do ovo de 16 espécies de Pentatomidae como exemplo de uso de caracteres de imaturos em filogenias” VIVIANA CAUDURO MATESCO Tese apresentada como parte dos requisitos para obtenção de grau de Doutor em Biologia Animal, área de concentração Biologia Comparada. ________________________________________ Prof. Dr. Augusto Ferrari (UFRGS) ________________________________________ Dra. Caroline Greve (CNPq ex-bolsista PDJ) ________________________________________ Prof. Dr. Cláudio José Barros de Carvalho (UFPR) ________________________________________ Profa. Dra. Jocelia Grazia (Orientadora) Porto Alegre, 05 de fevereiro de 2014. AGRADECIMENTOS À minha orientadora, Profa. Dra. Jocelia Grazia, pelos ensinamentos e por todas as oportunidades que me deu durante os treze anos em que estive no Laboratório de Entomologia Sistemática. Ao meu co-orientador, Prof. -

Identification, Biology, Impacts, and Management of Stink Bugs (Hemiptera: Heteroptera: Pentatomidae) of Soybean and Corn in the Midwestern United States
Journal of Integrated Pest Management (2017) 8(1):11; 1–14 doi: 10.1093/jipm/pmx004 Profile Identification, Biology, Impacts, and Management of Stink Bugs (Hemiptera: Heteroptera: Pentatomidae) of Soybean and Corn in the Midwestern United States Robert L. Koch,1,2 Daniela T. Pezzini,1 Andrew P. Michel,3 and Thomas E. Hunt4 1 Department of Entomology, University of Minnesota, 1980 Folwell Ave., Saint Paul, MN 55108 ([email protected]; Downloaded from https://academic.oup.com/jipm/article-abstract/8/1/11/3745633 by guest on 08 January 2019 [email protected]), 2Corresponding author, e-mail: [email protected], 3Department of Entomology, Ohio Agricultural Research and Development Center, The Ohio State University, 210 Thorne, 1680 Madison Ave. Wooster, OH 44691 ([email protected]), and 4Department of Entomology, University of Nebraska, Haskell Agricultural Laboratory, 57905 866 Rd., Concord, NE 68728 ([email protected]) Subject Editor: Jeffrey Davis Received 12 December 2016; Editorial decision 22 March 2017 Abstract Stink bugs (Hemiptera: Heteroptera: Pentatomidae) are an emerging threat to soybean and corn production in the midwestern United States. An invasive species, the brown marmorated stink bug, Halyomorpha halys (Sta˚ l), is spreading through the region. However, little is known about the complex of stink bug species associ- ated with corn and soybean in the midwestern United States. In this region, particularly in the more northern states, stink bugs have historically caused only infrequent impacts to these crops. To prepare growers and agri- cultural professionals to contend with this new threat, we provide a review of stink bugs associated with soybean and corn in the midwestern United States.