Economic Assessment of Herbicide Systems for Minimum-Tillage Peanuts1 John W
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2,4-Dichlorophenoxyacetic Acid
2,4-Dichlorophenoxyacetic acid 2,4-Dichlorophenoxyacetic acid IUPAC (2,4-dichlorophenoxy)acetic acid name 2,4-D Other hedonal names trinoxol Identifiers CAS [94-75-7] number SMILES OC(COC1=CC=C(Cl)C=C1Cl)=O ChemSpider 1441 ID Properties Molecular C H Cl O formula 8 6 2 3 Molar mass 221.04 g mol−1 Appearance white to yellow powder Melting point 140.5 °C (413.5 K) Boiling 160 °C (0.4 mm Hg) point Solubility in 900 mg/L (25 °C) water Related compounds Related 2,4,5-T, Dichlorprop compounds Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) 2,4-Dichlorophenoxyacetic acid (2,4-D) is a common systemic herbicide used in the control of broadleaf weeds. It is the most widely used herbicide in the world, and the third most commonly used in North America.[1] 2,4-D is also an important synthetic auxin, often used in laboratories for plant research and as a supplement in plant cell culture media such as MS medium. History 2,4-D was developed during World War II by a British team at Rothamsted Experimental Station, under the leadership of Judah Hirsch Quastel, aiming to increase crop yields for a nation at war.[citation needed] When it was commercially released in 1946, it became the first successful selective herbicide and allowed for greatly enhanced weed control in wheat, maize (corn), rice, and similar cereal grass crop, because it only kills dicots, leaving behind monocots. Mechanism of herbicide action 2,4-D is a synthetic auxin, which is a class of plant growth regulators. -

Decision Guidance Documents
OPERATION OF THE PRIOR INFORMED CONSENT PROCEDURE FOR BANNED OR SEVERELY RESTRICTED CHEMICALS IN INTERNATIONAL TRADE DECISION GUIDANCE DOCUMENTS Dinoseb and its salts and esters JOINT FAO/UNEP PROGRAMME FOR THE OPERATION OF PRIOR INFORMED CONSENT United Nations Environment Programme UNEP Food and Agriculture Organization of the United Nations OPERATION OF THE PRIOR INFORMED CONSENT PROCEDURE FOR BANNED OR SEVERELY RESTRICTED CHEMICALS IN INTERNATIONAL TRADE DECISION GUIDANCE DOCUMENTS Dinoseb and its salts and esters JOINT FAO/UNEP PROGRAMME FOR THE OPERATION OF PRIOR INFORMED CONSENT Food and Agriculture Organization of the United Nations United Nations Environment Programme Rome - Geneva 1991 DISCLAIMER The inclusion of these chemicals in the Prior Informed Consent Procedure is based on reports of control action submitted to the United Nations Environment Programme (UNEP) by participating countries, and which are presently listed in the UNEP-International Register of Potentially Toxic Chemicals (IRPTC) database on Prior Informed Consent. While recognizing that these reports from countries are subject to confirmation, the FAO/UNEP Joint Working Group of Experts on Prior Informed Consent have recommended that these chemical be included in the Procedure. The status of these chemicals will be reconsidered on the basis of such new notifications as may be made by participating countries from time to time. The use of trade names in this document is primarily intended to facilitate the correct identification of the chemical. It is not intended to imply approval or disapproval of any particular company. As it is not possible to include all trade names presently in use, only a number of commonly used and published trade names have been included here. -

Herbicides to Isolated Thylakoid Membranes and Intact
Comparative Binding of Photosystem II — Herbicides to Isolated Thylakoid Membranes and Intact Green Algae Henrik Laasch, Klaus Pfister, and Wolfgang Urbach Botanisches Institut der Universität Würzburg, Mittlerer Dallenbergweg 64, D-8700 Würzburg, Bundesrepublik Deutschland Z. Naturforsch. 36 c, 1041-1049 (1981); received July 13, 1981 Herbicides, Herbicide Binding, Photosynthesis, Thylakoids, Algae The binding of the photosystem II herbicides diuron (DCMU), atrazine (s-triazine), ioxynil and dinoseb (substituted phenols) to isolated spinach thylakoids was saturated in less than 2 min in the dark. In intact cells of the green alga Ankistrodesmus b. it took 10 to 20 min to reach the binding equilibrium. Binding affinity of diuron, atrazine, dinoseb, measured as equilibrium binding constants, was found to be comparable in isolated thylakoids and intact algal cells. For ioxynil, reduced binding affinity was observed in algae. The concentration of binding sites in thylakoids and intact cells was determined to be 300-500 chl/inhibitor binding site, suggesting a 1:1 stoichiometry between bound herbicide and electron transport chains. In intact cells only the phenol herbicides ioxynil and dinoseb showed increased concentrations of binding sites. Strong correlation of herbicide binding and inhibition of electron transport was found for diuron in isolated thylakoids and intact cells. In thylakoids this is valid also for atrazine and dinoseb. For ioxynil a difference between the amount of binding and inhibition was found. This correlation of herbicide binding and inhibition proves that binding specifically occurs at the inhibition site at photosystem H. In addition to the specific binding, for all four herbicides studied, (except for ioxynil in thylakoids) unspecific binding was observed in thylakoids as well as in algae, which was not related to inhibition. -

Ph DEPENDENCE of the EFFECTS of DIURON, ATRAZINE and DINOSEB on the LUMINESCENT PROPERTIES of THYLAKOID MEMBRANES
BULG. J. PLANT PHYSIOL., 2001, 27(1–2), 85–100 85 pH DEPENDENCE OF THE EFFECTS OF DIURON, ATRAZINE AND DINOSEB ON THE LUMINESCENT PROPERTIES OF THYLAKOID MEMBRANES Petar Lambrev, Vassilij Goltsev* Department of Biophysics and Radiobiology, Biological Faculty, University of Sofia “St. Kliment Ohridski” Received May 17, 2001 Summary. The effect of the external pH in the range of 5.5 to 8.0 on the activity of three PSII herbicides (diuron, atrazine and dinoseb) was studied in isolated thylakoid membranes by means of prompt and delayed fluores- cence induction kinetics. Well-expressed pH dependence was established for prompt fluorescence parameters (Fv/Fm, Fi/Fm) as well as for delayed fluores- cence parameters (I1, I2). Two types of pH dependence of the herbicide effect were observed. The apparent resistance to herbicides of the urea/triazine type (diuron and atrazine) was found to be maximal at pH 6–6.5, although the amplitude of the herbicide effect was different between diuron and atrazine. The pH dependences for thylakoids treated with the phenolic herbicide, dino- seb, showed a minimum in the herbicide effect at pH 7.5. The mechanism by which pH influences the herbicide sensitivity of thylakoid membranes is supposed to involve pH-induced conformational changes of the QB-binding site on the D1 protein of PSII, resulting in alteration of the affinity to the herbicide molecule. The conformation of the QB-site at a cer- tain pH is characterized by maximal herbicide resistance. The optimal value of pH depends on the type of herbicide, specifically the location of the H- bond between the herbicide molecule and D1. -

Leaching of Dinoseb and Metribuzin from Potato Fields in New Brunswick P
Leaching of dinoseb and metribuzin from potato fields in New Brunswick P. MILBURN1, H. O'NEILL2, C. GARTLEY3, T. POLLOCK2, J.E. RICHARDS1 and H. BAILEY2 1Agriculture Canada Research Station, P.O. Box 20280, Fredericton, NB, Canada E3B 4Z7; Environment Canada, Inland Waters Directorate, P.O. Box 861, Moneton, NB, Canada E1C 8N6; and JSoil and Water Section, New Brunswick Department ofAgriculture, P.O. Box 6000, Fredericton, NB, Canada E3B 5H1. Received 12 February 1990; accepted 7March 1991 Milbum, P.,O'Neill, H., Gartley, C, Pollock, T., Richards, J.E. and in Iowa and Minnesota alone. He furthernoted that pesticides Bailey, H. 1991. Leaching of dinoseb andmetribuzin from potato havebeen detected in winterand spring water samples, prior fields in New Brunswick. Can.Agric.Eng.33:197-204. Tiledrainage to new application, indicating that several pesticides are per waters from five systematically tiled, commercial potato fields in sisting in groundwater year-round. northwestern New Brunswick were analyzed for the herbicides Approximately 36 000 metric tonnes of pesticide active metribuzin and dinoseb from April 1987 to April 1989. Tile drain ingredient were sold in Canada in 1985 (Pierce and Wong outflowrate and volume were also recorded. Dinoseb and metribuzin 1988). Frank et al. (1987b) reported on sampling 359 rural were detected in tile outflow both during the year of application and during the subsequent spring melt period, butat concentrations sub wells in Ontario for suspectedpesticide contamination where stantially less than maximum acceptable concentrations (MAC) for the cited causes were spills, spray drift, or surface runoff drinking water published by Health and Welfare Canada. -

PLANTS TOLERANT to HPPD INHIBITOR HERBICIDES HPPD-Inhibitorherbizid-Resistente Pflanzen Plantes Tolérantes À Des Herbicides Inhibant L’HPPD
(19) TZZ _¥Z_T (11) EP 2 516 630 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: C12N 9/02 (2006.01) C12N 15/82 (2006.01) 15.11.2017 Bulletin 2017/46 A01H 5/00 (2006.01) A01H 5/10 (2006.01) (21) Application number: 10793270.9 (86) International application number: PCT/EP2010/070561 (22) Date of filing: 22.12.2010 (87) International publication number: WO 2011/076877 (30.06.2011 Gazette 2011/26) (54) PLANTS TOLERANT TO HPPD INHIBITOR HERBICIDES HPPD-Inhibitorherbizid-resistente Pflanzen Plantes tolérantes à des herbicides inhibant l’HPPD (84) Designated Contracting States: • HAIN, Ruediger AL AT BE BG CH CY CZ DE DK EE ES FI FR GB 60594 Frankfurt (DE) GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR (56) References cited: WO-A1-2009/144079 WO-A2-96/38567 (30) Priority: 23.12.2009 EP 09015984 29.12.2009 US 290573 P • DATABASE EMBL [Online] 7 February 2006 10.11.2010 EP 10190655 (2006-02-07), "Synechococcus sp. JA-3-3Ab 10.11.2010 US 412093 P putative 4-hydroxyphenylpyruvate dioxygenase", XP002583274, retrieved from EBI (43) Date of publication of application: accession no. EMBL:ABC98696 Database 31.10.2012 Bulletin 2012/44 accession no. ABC98696 • DATABASE EMBL [Online] 7 February 2006 (73) Proprietor: Bayer Intellectual Property GmbH (2006-02-07), "Synechococcus sp. 40789 Monheim (DE) JA-2-3B’a(2-13) 4-hydroxyphenylpyruvate dixygenase, putative", XP002583275, retrieved (72) Inventors: from EBI accession no. -

Dermal Absorption of 2,4-D Is Underestimated EPA Used an Outdated Study to Estimate Dermal Absorption of 2,4-D
August 23, 2004 Environmental Protection Agency Public Information and Records Integrity Branch (7502C) Office of Pesticide Programs 1200 Pennsylvania Ave., NW Washington, DC 20460-0001 RE: 2,4-D Risk Assessment; Docket ID No. OPP-2004-0167 Submitted electronically to [email protected] Dear Sir or Madam: We submit the following comments on behalf of Beyond Pesticides; Natural Resources Defense Council; TEDX, Inc. (The Endocrine Disruption Exchange); Pesticide Action Network North America; Northwest Coalition for Alternatives to Pesticides; Washington Toxics Coalition; Coalition for Health, Environment and Economic Rights; Cancer Prevention Coalition; The Breast Cancer Fund; Alliance for Healthy Homes; Farmworker Justice Fund, Inc.; Agricultural Resources Center; Institute for Agriculture and Trade Policy; Roseland Organic Farms; Safer Pest Control Project; Defenders of Wildlife; California Safe Schools; Advocates for Environmental Human Rights; Californians for Alternatives to Toxics; New York Public Interest Research Group; New Jersey Environmental Federation; Wyoming Outdoor Council; Alaska Community Action on Toxics; Ecology Center; Citizens' Environmental Coalition; Environmental Research Foundation; Clean Water Action; Toxics Action Center; Informed Choices; National Center for Environmental Health Strategies, Inc.; The Coalition for Alternatives to Pesticides; Texans for Alternatives to Pesticides; Jack B. Richman Environmental Coalition; Colorado Pesticide Network; Grassroots Coalition; Women's Voices for the Earth; Grassroots Environmental Education; No Spray Coalition, Nashville; Students for Bhopal; Citizens' Campaign for the Environment; Connecticut Coalition for EPA 2,4-D Risk Assessment Public Comments 2 Environmental Justice; and the Coalition for Environmentally Safe Communities. (See page 33 to 36 for signatures.) None of our organizations has any direct or indirect financial or fiduciary interest in the manufacture or sale of 2,4-dichlorophenoxyacetic acid, amine salts, esters, or any related alkylchlorophenoxy chemicals. -

57518 Federal Register / Vol. 61, No. 216 / Wednesday, November 6, 1996 / Rules and Regulations
57518 Federal Register / Vol. 61, No. 216 / Wednesday, November 6, 1996 / Rules and Regulations ENVIRONMENTAL PROTECTION implement certain pollution prevention, (BPT), §§ 455.43 and 455.63 (BCT), and AGENCY recycle and reuse practices. Facilities §§ 455.44 and 455.64 (BAT) are choosing and implementing the established in the National Pollutant 40 CFR Part 455 pollution prevention alternative will Discharge Elimination System (NPDES) receive a discharge allowance. permits. [FRL±5630±9] The final rule will benefit the ADDRESSES: For additional technical RIN 2040±AC21 environment by removing toxic information write to Ms. Shari H. pollutants (pesticide active ingredients Zuskin, Engineering & Analysis Division Pesticide Chemicals Category, and priority pollutants) from water (4303), U.S. EPA, 401 M Street SW, Formulating, Packaging and discharges that have adverse effects on Washington, D.C. 20460 or send e-mail Repackaging Effluent Limitations human health and aquatic life. EPA has to: [email protected] or call Guidelines, Pretreatment Standards, estimated the compliance costs and at (202) 260±7130. For additional and New Source Performance economic impacts expected to result economic information contact Dr. Lynne Standards from the Zero Discharge/Pollution Tudor at the address above or by calling Prevention Alternative (i.e., Zero/P2 AGENCY: Environmental Protection (202) 260±5834. Agency. Alternative). The Agency has determined that the Zero/P2 Alternative The complete record (excluding ACTION: Final rule. will result in a similar removal of toxic confidential business information) for this rulemaking is available for review SUMMARY: This final regulation limits pound equivalents per year at EPA's Water Docket; 401 M Street, the discharge of pollutants into (approximately 7.6 million toxic pound SW, Washington, DC 20460. -

List of Banned Pesticides and Pesticides Watchlist
LIST OF BANNED PESTICIDES AND PESTICIDES WATCHLIST Version 1.0 0 Translations of this document are available in electronic format on the UTZ website: www.utz.org/resource-library.org Please send your comments or suggestions to: [email protected] [email protected] [email protected] [email protected] Or via regular mail to: UTZ Standard and Certification Department De Ruyterkade 6 bg 1013 AA Amsterdam The Netherlands © UTZ 2015 No part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying, recording or otherwise without full attribution. 1 www.utz.org Abbreviations EPA Environmental Protection Agency EU European Union GHS Globally Harmonized System HHP Highly Hazardous Pesticides ISEAL Alliance International Social and Environmental Accreditation and Labelling Alliance PAN Pesticide Action Network PIC Prior Informed Consent POP Persistent Organic Pollutant UNEP United Nations Environmental Program WHO World Health Organization Definitions Active ingredient The chemical substance or component of a pesticide product that can kill, repel, attract, mitigate or otherwise control a pest (as opposed to “inert ingredients” such as water, solvents, emulsifiers, surfactants, clay and propellants). Banned Pesticide A pesticide all uses of which have been prohibited by final regulatory action, in order to protect human health or the environment. Bee Colony Pathological condition affecting a large number of honeybee colonies in which worker Collapse Disorder bees abruptly disappear. Bioaccumulative Characterizes substances thatconcentrate in fatty tissue and tend to build up to much higher concentrations in humans and other organisms. They are also more likely to transfer and accumulate up the food chain. -
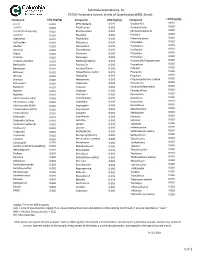
P2220 MDL Sheet.Xlsx
Columbia Laboratories, Inc P2750 Herbicides Screen, Limits of Quantitation (MDL Sheet) Compound LOQ (mg/kg) Compound LOQ (mg/kg) Compound LOQ (mg/kg) 0.010 2,4-D 0.010 EPTC (Eptam) 0.010 Oxyfluorfen 0.010 2,4-DB 0.010 Ethalfluralin 0.010 Pendimethalin 0.010 2,4-DP (Dichlorprop) 0.010 Ethofumesate 0.010 Pentachlorophenol 0.010 2,4,5-TP 0.010 Fluazifop 0.010 Picloram 0.010 Acetochlor 0.020 Fluchloralin 0.010 Phenmedipham 0.010 Acifluorfen 0.010 Flufenacet 0.010 Pinoxaden 0.010 Alachlor 0.020 Flumioxazin 0.010 Prodiamine 0.010 Ametryn 0.010 Fluometuron 0.010 Profluralin 0.010 Aspon 0.010 Fluridone 0.010 Prometon 0.010 Atrazine 0.010 Fluroxypyr 0.010 Prometryn 0.010 Atrazine-desethyl 0.010 Fluthiacet Methyl 0.010 Pronamide (Propyzamide) 0.010 Benfluralin 0.010 Fomesafen 0.010 Propachlor 0.010 Benoxacor 0.010 Foramsulfuron 0.010 Propanil 0.010 Bentazon 0.010 Halosulfuron-methyl 0.010 Propazine 0.010 Bifenox 0.010 Haloxyfop 0.010 Propham 0.010 Bromacil 0.020 Hexazinone 0.010 Propoxycarbazone sodium 0.010 Bromoxynil 0.010 Imazamox 0.010 Prosulfuron 0.010 Butachlor 0.010 Imazapic 0.010 Pyridate (Metabolite) 0.010 Butralin 0.020 Imazapyr 0.010 Pyroxasulfone 0.010 Butylate 0.010 Imazaquin 0.010 Pyroxsulam 0.010 Carfentrazone-ethyl 0.010 Imazethaphyr 0.010 Quinclorac 0.010 Chlorimuron-ethyl 0.010 Indaziflam 0.010 Quizalofop 0.010 Chlornitrofen (CNP) 0.020 Isopropalin 0.010 Rimsulfuron 0.010 Chlorpropham (CIPC) 0.010 Isoproturon 0.010 Sebuthylazine 0.010 Chlorsulfuron 0.010 Isoxaben 0.010 Sethoxydim 0.010 Clethodim 0.010 Isoxaflutole 0.010 Simazine -
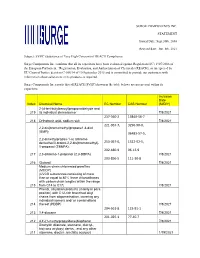
SURGE COMPONENTS INC. STATEMENT Issued Date: Sept.30Th, 2016 Revised Date: Jun. 8Th, 2021 Subject: SVHC (Substance of Very High
SURGE COMPONENTS INC. STATEMENT Issued Date: Sept.30th, 2016 Revised date: Jun. 8th, 2021 Subject: SVHC (Substance of Very High Concern) of REACH Compliance Surge Components Inc. confirms that all its capacitors have been evaluated against Regulation (EC) 1907/2006 of the European Parliament, “Registration, Evaluation, and Authorization of Chemicals (REACH), as interpreted by EU Court of Justice decision C-106/14 of 10 September 2015 and is committed to provide our customers with information about substances in its products as required. Surge Components Inc.reports that all REACH SVHC(shown in the table below) are not present within its capacitors. Inclusion Date Index Chemical Name EC Number CAS Number (M/D/Y) 2-(4-tert-butylbenzyl)propionaldehyde and 219 its individual stereoisomer 7/8/2021 237-560-2 13840-56-7 218 Orthoboric acid, sodium salt 7/8/2021 221-967-7, 3296-90-0, 2,2-bis(bromomethyl)propane1,3-diol (BMP); 36483-57-5, 2,2-dimethylpropan-1-ol, tribromo derivative/3-bromo-2,2-bis(bromomethyl)- 253-057-0, 1522-92-5, 1-propanol (TBNPA); 202-480-9 96-13-9 217 2,3-dibromo-1-propanol (2,3-DBPA) 7/8/2021 203-856-5 111-30-8 216 Glutaral 7/8/2021 Medium-chain chlorinated paraffins (MCCP) (UVCB substances consisting of more than or equal to 80% linear chloroalkanes with carbon chain lengths within the range 215 from C14 to C17) 7/8/2021 Phenol, alkylation products (mainly in para position) with C12-rich branched alkyl chains from oligomerisation, covering any individual isomers and/ or combinations 214 thereof (PDDP) 7/8/2021 204-661-8 123-91-1 213 1,4-dioxane 7/8/2021 201-025-1 77-40-7 212 4,4'-(1-methylpropylidene)bisphenol 7/8/2021 Dioctyltin dilaurate, stannane, dioctyl-, bis(coco acyloxy) derivs., and any other 211 stannane, dioctyl-, bis(fatty acyloxy) 1/19/2021 derivs. -

Pesticide Use in US Agriculture
United States Department of Agriculture Economic Research Pesticide Use in U.S. Agriculture: Service Economic 21 Selected Crops, 1960-2008 Information Bulletin Number 124 Jorge Fernandez-Cornejo May 2014 Richard Nehring Craig Osteen Seth Wechsler Andrew Martin Alex Vialou United States Department of Agriculture Economic Research Service www.ers.usda.gov Access this report online: www.ers.usda.gov/publications/eib-economic-information-bulletin/eib124.aspx Download the charts contained in this report: • Go to the report’s index page www.ers.usda.gov/publications/ eib-economic-information-bulletin/eib124.aspx • Click on the bulleted item “Download eib124.zip” • Open the chart you want, then save it to your computer Recommended citation format for this publication: Fernandez-Cornejo, Jorge, Richard Nehring, Craig Osteen, Seth Wechsler, Andrew Martin, and Alex Vialou. Pesticide Use in U.S. Agriculture: 21 Selected Crops, 1960-2008, EIB-124, U.S. Department of Agriculture, Economic Research Service, May 2014. Cover images: Shutterstock. Use of commercial and trade names does not imply approval or constitute endorsement by USDA. The U.S. Department of Agriculture (USDA) prohibits discrimination in all its programs and activities on the basis of race, color, national origin, age, disability, and, where applicable, sex, marital status, familial status, parental status, religion, sexual orientation, genetic information, political beliefs, reprisal, or because all or a part of an individual’s income is derived from any public assistance program. (Not all prohibited bases apply to all programs.) Persons with disabilities who require alternative means for communication of program information (Braille, large print, audiotape, etc.) should contact USDA’s TARGET Center at (202) 720-2600 (voice and TDD).