Long-Term Clinical Course in Eyes with Peters Anomaly
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Lid and Lash Conditions
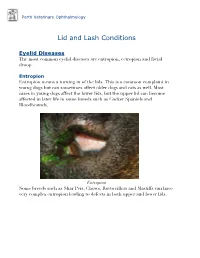
Perth Veterinary Ophthalmology Lid and Lash Conditions Eyelid Diseases The most common eyelid diseases are entropion, ectropion and facial droop. Entropion Entropion means a turning in of the lids. This is a common complaint in young dogs but can sometimes affect older dogs and cats as well. Most cases in young dogs affect the lower lids, but the upper lid can become affected in later life in some breeds such as Cocker Spaniels and Bloodhounds. Entropion Some breeds such as Shar Peis, Chows, Rottweillers and Mastiffs can have very complex entropion leading to defects in both upper and lower lids. A Shar Pei with severe upper and lower lid entropion Entropion is painful and can be potentially blinding. The rolling in of the lid leads to hair coming into contact with the cornea, leading to pain, ulceration and scarring (which can affect vision). In severe cases this can even lead to perforation of the eye. There are many causes of entropion. It can be primary or secondary to other problems affecting the lids (such as ectopic cilia, distichiasis etc. - see below). Some possible causes include the lid being too long, the lid being too tight, instability of the lateral canthus (outer cornea of the eyelids), misdirection of the lateral canthal tendon, brachycephalic anatomy (big eyes and short nose - e.g. Pekingese, Pugs, Shih Tsus, Persian cats etc.), diamond eye defects, loose or too much skin, facial droop etc. Often these cases are referred to a veterinary ophthalmologist for proper assessment and treatment to provide the best outcome. Entropion requires surgical correction. -

Eleventh Edition
SUPPLEMENT TO April 15, 2009 A JOBSON PUBLICATION www.revoptom.com Eleventh Edition Joseph W. Sowka, O.D., FAAO, Dipl. Andrew S. Gurwood, O.D., FAAO, Dipl. Alan G. Kabat, O.D., FAAO Supported by an unrestricted grant from Alcon, Inc. 001_ro0409_handbook 4/2/09 9:42 AM Page 4 TABLE OF CONTENTS Eyelids & Adnexa Conjunctiva & Sclera Cornea Uvea & Glaucoma Viitreous & Retiina Neuro-Ophthalmic Disease Oculosystemic Disease EYELIDS & ADNEXA VITREOUS & RETINA Blow-Out Fracture................................................ 6 Asteroid Hyalosis ................................................33 Acquired Ptosis ................................................... 7 Retinal Arterial Macroaneurysm............................34 Acquired Entropion ............................................. 9 Retinal Emboli.....................................................36 Verruca & Papilloma............................................11 Hypertensive Retinopathy.....................................37 Idiopathic Juxtafoveal Retinal Telangiectasia...........39 CONJUNCTIVA & SCLERA Ocular Ischemic Syndrome...................................40 Scleral Melt ........................................................13 Retinal Artery Occlusion ......................................42 Giant Papillary Conjunctivitis................................14 Conjunctival Lymphoma .......................................15 NEURO-OPHTHALMIC DISEASE Blue Sclera .........................................................17 Dorsal Midbrain Syndrome ..................................45 -

The Management of Congenital Malpositions of Eyelids, Eyes and Orbits
Eye (\988) 2, 207-219 The Management of Congenital Malpositions of Eyelids, Eyes and Orbits S. MORAX AND T. HURBLl Paris Summary Congenital malformations of the eye and its adnexa which are multiple and varied can affect the whole eyeball or any part of it, as well as the orbit, eyelids, lacrimal ducts, extra-ocular muscles and conjunctiva. A classification of these malformations is presented together with the general principles of treatment, age of operating and surgical tactics. The authors give some examples of the anatomo-clinical forms, eyelid malpositions such as entropion, ectropion, ptosis, levator eyelid retraction, medial canthus malposition, congenital eyelid colobomas, and congenital orbital abnormalities (Craniofacial stenosis, orbi tal plagiocephalies, hypertelorism, anophthalmos, microphthalmos and cryptophthalmos) . Congenital malformations of the eye and its as echography, CT-scan and NMR, enzymatic adnexa are multiple and varied. They can work-up or genetic studies (Table I). affect the whole eyeball or any part of it, as Surgical treatment when feasible will well as the orbit, eyelids, lacrimal ducts extra encounter numerous problems; age will play a ocular muscles and conjunctiva. role, choice of a surgical protocol directly From the anatomical point of view, the fol related to the existing complaints, and coop lowing can be considered. eration between several surgical teams Position abnormalities (malpositions) of (ophthalmologic, plastic, cranio-maxillo-fac one or more elements and formation abnor ial and neurosurgical), the ideal being to treat malities (malformations) of the same organs. Some of these abnormalities are limited to Table I The manag ement of cong enital rna/positions one organ and can be subjected to a relatively of eyelid s, eyes and orbits simple and well recognised surgical treat Ocular Findings: ment. -

Entropion, Ectropion and Ptosis Surgery
Patient Information Entropion, Ectropion and Ptosis Surgery ENTROPION is a lid condition in which the eyelid BEFORE YOUR OPERATION becomes lax and turns inwards. This causes the eyelashes to rub and irritate the eye making it red, uncomfortable and watery. The lower lid can Do I take my normal medication? be pulled back into the right position temporarily with a piece of tape placed between the lower Please stop aspirin 1 week prior to surgery lid and your cheek. An operation is needed to with permission from your GP. If you take any correct this condition permanently. medication to thin your blood e.g. warfarin as prescribed medication, please contact your GP ECTROPION is a lid condition in which the eyelid two weeks before your operation for further becomes lax and turns outwards. This may make instructions - telephone 01392 406013. the eyes water. This is treated by an operation to Otherwise take your normal medication and tighten the eyelid. bring them into hospital with you. There is a small risk that the conditions above may reoccur and require more than one Can I eat and drink normally? operation. There is also a small risk of bleeding and infection. Yes. Please have breakfast before morning surgery and a light lunch before afternoon PTOSIS (pronounced ‘Toesis’) in adults is a surgery. condition where the upper eyelid stretches and begins to droop. This can look unsightly and in Do I need to wear anything special? severe cases can affect the eyesight. It is treated Yes. Wear comfortable loose fitting clothes by an operation which tightens the muscle that as you may be asked to change into a theatre opens your upper eyelid. -

Surgery Prioritization Strategy: Codes for Typical Ophthalmic Procedures
Surgery Prioritization Strategy: Codes for Typical Ophthalmic Procedures Emergent Condition/Service CPT code(s) Endophthalmitis 67015 Aspiration or release of vitreous, subretinal or choroidal fluid, pars plana approach (posterior sclerotomy) 65800 Paracentesis of anterior chamber of eye (separate procedure); with removal of aqueous 67028 Intravitreal injection of a pharmacologic agent (separate procedure) Note: 67028 is bundled with both 67015 and 65800 Open globe, disruption of operative 65275 Repair of laceration; cornea, nonperforating, with or without removal foreign body incision 65280 Repair of laceration; cornea and/or sclera, perforating, not involving uveal tissue 65285 Repair of laceration; cornea and/or sclera, perforating, with reposition or resection of uveal tissue 65286 Repair of laceration; application of tissue glue, wounds of cornea and/or sclera 66250 Revision or repair of operative wound of anterior segment, any type, early or late, major or minor procedure Cantholysis/canthotomy 67715 Canthotomy (separate procedure) Intraocular foreign body 65235 Removal of foreign body, intraocular; from anterior chamber of eye or lens 65260 Removal of foreign body, intraocular; from posterior segment, magnetic extraction, anterior or posterior route 65265 Removal of foreign body, intraocular; from posterior segment, nonmagnetic extraction 67413 Orbitotomy without bone flap (frontal or transconjunctival approach); with removal of foreign body Canalicular lacerations 68700 Plastic repair of canaliculi 67950 Canthoplasty (reconstruction -

Clinical Study of Secondary Intraocular Lens Implantation
International Journal of Science and Research (IJSR) ISSN (Online): 2319-7064 Index Copernicus Value (2013): 6.14 | Impact Factor (2015): 6.391 Clinical Study of Secondary Intraocular Lens Implantation Dr. Kshitija Panditrao1, Dr. R. R. Naik2 Department of Ophthalmology, PDVVPF's Medical College, Ahmednagar, 414111, Maharashtra, India Abstract: Background: Secondary implantation of lens is an insertion of a lens in any eye rendered aphakic by trauma or previous surgery. Aims and objectives: To study and analyse the result of secondary IOL implantation. Materials and methods: This is a hospital based clinical study of 12 patients with aphakia attending the ophthalmology out patient department of our Hospital. These cases were treated surgically with secondary iol implantation and then results were analysed with respect to study of indications for secondary IOL implantation, the functional outcome following secondary IOL implantation and to understand the reason for poor visual acuity following secondary IOL implantation. Result: 12 patients underwent secondary IOL implantation. All were in the age group of 61 to 70. 83.33% of patients had pseudophakia as the status of the other eye and 16.66% had aphakia in fellow eye. Monoocular aphakia with pseudophakia in fellow eye was the indication for secondary IOL implantation. 41% underwent ACIOL implantation, 41% underwent Scleral fixative and 16% underwent PCIOL implant in sulcus. 75% eyes had BCVA 6/12 or better and 25% had BCVA 6/24 or better. Striate keratopathy in 25%, uveitis in 33.33% and Cystoid macular edema in 8.33%. Interpretation and conclusion: Majority of patients seeking IOL implantation have monocular aphakia with good vision in the fellow eye. -

Contact Lens Options and Fitting Strategies for the Management of the Irregular Cornea
6/23/2017 CONTACT LENS OPTIONS AND FITTING STRATEGIES FOR THE MANAGEMENT OF THE IRREGULAR CORNEA DAVID I. GEFFEN, OD, FAAO David I Geffen, OD, FAAO Consultant/Advisor/Speaker Accufocus Shire Alcon Tear Lab AMO Tear Science Annidis Bausch + Lomb TLC Vision Bruder Healthcare EyeBrain Optovue Revision Optics 1 6/23/2017 Irregular Cornea Contact Lens Options Standard Soft Lenses Custom Keratoconic Soft Lenses Corneal Gas Permeable Lenses Intra-Limbal Gas Permeable Lenses Piggyback and Recess Systems Scleral Gas Permeable Lenses Hybrid Lenses Types of Irregular Corneas DEGENERATIONS DYSTROPHIES • Keratoconus • Cogan’s dystrophy • Bowman’s dystrophy • Keratoglobus • Granular corneal dystrophy • Pellucid marginal degeneration • Lattice corneal dystrophy • Terrien’s marginal degeneration • Meesmann’s corneal dystrophy • Salzmann’s nodular degeneration CORNEAL SCARRING • Ehlers-Danlos syndrome • After infection AFTER SURGERY • After trauma • Cornea transplant (PK, PKP) • Radial keratotomy (RK) • Photorefractive keratectomy (PRK) • Phototherapeutic keratectomy (PTK) • Epikeratophakia • LASIK 2 6/23/2017 CL Options: Soft Lenses Advantages: . Comfort . Centration (draping) . Corneal Protection Limitations: . Vision (due to draping effect) . Dehydration . Hypoxia /microbial contamination CL Options: Custom Soft KC Lenses Hydrokone (Visionary Optics) NovaKone (Alden) Kerasoft (dist. By B&L) Soft K (Acculens & Advanced Vision, & SLIC Labs) Continental Kone (Continental) Keratoconus lens (Gelflex) Soflex (Marietta) -

Eyelid Malposition After Orbital Fracture Surgery
7 Review Article Page 1 of 7 Eyelid malposition after orbital fracture surgery Yue Xing1,2, Yongwei Guo3,4, Xia Ding, Jin Li1,2, Ming Lin1,2 1Department of Ophthalmology, Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China; 2Shanghai Key Laboratory of Orbital Diseases and Ocular Oncology, Shanghai, China; 3Eye Center, Second Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, China; 4Department of Ophthalmology, University of Cologne, Faculty of Medicine and University Hospital Cologne, Cologne, Germany Contributions: (I) Conception and design: M Lin, J Li; (II) Administrative support: M Lin; (III) Provision of study materials or patients: M Lin, J Li; (IV) Collection and assembly of data: M Lin, Y Xing, Y Guo; (V) Data analysis and interpretation: All authors; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors. Correspondence to: Ming Lin, MD; Jin Li, MD. Department of Ophthalmology, Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, 639 Zhizaoju Road, Shanghai 200011, China. Email: [email protected]; [email protected]. Abstract: Orbital fractures generally do not cause eyelid malposition. Studies have shown that mostly eyelid malposition is mainly due to the choice of surgical approaches of orbital fracture repair. Approaches are divided into transcutaneous and transconjunctival ones. The application of orbital fracture approaches depends on fractures’ range and the surgeons’ preferences. Eyelid malposition after orbital fracture surgery is not only an aesthetic concern but also a functional complication, which will cause eyes discomfort, such as corneal exposure and ocular irritation. Some patients may have multiple types of eyelid malposition. -

Botulinum Toxin for the Temporary Treatment of Involutional Lower Lid Entropion: a Clinical and Morphological Study
BOTULINUM TOXIN FOR THE TEMPORARY TREATMENT OF INVOLUTIONAL LOWER LID ENTROPION: A CLINICAL AND MORPHOLOGICAL STUDY 1 3 l D. H. W. STEEL\ H. B. HOH , , R. A. HARRAD and C. R. COLLINS2 Bristol SUMMARY treatment and waiting lists for surgery are the norm. Purpose: A prospective study was designed to evaluate During this waiting period corneal complications may the use of botulinum toxin as a temporary treatment in occur. A variety of temporary conservative measures patients awaiting surgical repair for involutional entro such as lid taping and lubricant ointment are used to pion and to compare its use with lid taping. alleviate symptoms and prevent ocular complications Methods: Botulinum toxin was administered to 30 whilst awaiting surgery. A retrospective study of patients with involutional entropion (35 eyelids). senile entropion at our hospital highlighted problems These patients had all previously been using lid taping with the use of taping and an alternative temporary 1 and lubricant ointment as a temporary measure whilst treatment was sought. awaiting lid surgery. Patients' symptoms and signs were The successful use of botulinum toxin in involu assessed before and after toxin injection. The date of tional entropion has been reported2,3 but has not entropion recurrence was recorded. Eyelid tissue from been generally accepted as a form of treatment. A 8 patients treated with toxin and 3 control patients who pilot study confirmed that the technique was had not been given toxin was obtained after surgical acceptable to patients and produced encouraging entropion repair and examined histologically to ensure results.1 We designed a prospective study to compare the botulinum toxin had no potential detrimental the use of botulinum toxin in the temporary effects on the results of surgery. -

Surgical Correction of Hallermann-Streiff Syndrome: a Case Report of Esotropia, Entropion, and Blepharoptosis
Korean J Ophthalmol 2011;25(2):142-145 pISSN: 1011-8942 eISSN: 2092-9382 DOI: 10.3341/kjo.2011.25.2.142 Case Report Surgical Correction of Hallermann-Streiff Syndrome: A Case Report of Esotropia, Entropion, and Blepharoptosis Won-Kyung Cho, Joo Wan Park, Mi Ra Park Department of Ophthalmology and Visual Science, Yeouido St. Mary’s Hospital, The Catholic University of Korea School of Medicine, Seoul, Korea We report a case of surgical treatment for Hallermann-Streiff syndrome in a patient with ocular manifestations of esotropia, entropion, and blepharoptosis. A 54-year-old man visited Yeouido St. Mary’s Hospital complaining of oc- ular discomfort due to cilia touching the corneas of both eyes for several years. He had a bird-like face, pinched nose, hypotrichosis of the scalp, mandibular hypoplasia with forward displacement of the temporomandibular joints, a small mouth, and proportional short stature. His ophthalmic features included sparse eyelashes and eyebrows, microphthalmia, nystagmus, lower lid entropion in the right eye, and upper lid entropion with blepharoptosis in both eyes. There was esodeviation of the eyeball of more than 100 prism diopters at near and distance, and there were limitations in ocular movement on lateral gaze. The capsulopalpebral fascia was repaired to treat the right lower lid entropion, but an additional Quickert suture was required to prevent recurrence. Blepharoplasty and levator palpe- brae repair were performed for blepharoptosis and dermatochalasis. Three months after lid surgery, the right medial rectus muscle was recessed 7.5 mm, the left medial rectus was recessed 7.25 mm, and the left lateral rectus muscle was resected 8.0 mm. -

Blepharoplasty
ASPS Recommended Insurance Coverage Criteria for Third-Party Payers Blepharoplasty BACKGROUND Laterally, it is attached to the tarsus of the upper and Blepharoplasty is performed for both functional and lower eyelids. The lateral canthal tendon is attached to aesthetic reasons. the margin of the frontosphenoidal process of the zygomatic bone, and passes medial to the lateral Functional issues include ptosis, floppy eyelid syndrome, commisure of the eyelids, where it divides into two slips, blepharochalasis, dermatochalasis, herniated orbital fat, which are attached to the margins of the upper and lower and visual field obstructions. tarsi. The lacrimal glands are paired, almond-shaped glands, one for each eye, that secrete the aqueous layer of Aesthetic reasons include a desire for a more youthful the tear film. They are situated in the upper-outer and less fatigued appearance or improvement in aesthetic portion of each orbit, in the lacrimal fossa of the orbit appearance of the eyes. formed by the frontal bone. Blepharoplasty can be performed in combination with The lower eyelid is comprised of skin, orbicularis muscle, other procedures such as a browlift, facelift, or facial orbital septum, capsulopalpebral fascia, tarsus, and resurfacing. This may be done to restore or improve conjunctiva. The orbicularis has the same divisions as the function or facial expression as well as for aesthetic upper eyelid. In the lower eyelid, the orbital septum reasons. serves the same purpose. There are three lower eyelid fat compartments: nasal, central and temporal. The temporal Blepharoplasty, blepharoptosis repair, or brow lift is compartment may have more than one component. considered cosmetic and not medically necessary when performed to improve an individual’s DEFINITIONS appearance in the absence of any physical signs and Blepharoplasty is a procedure on the eyelid or eyelids to symptoms of functional abnormalities. -

Blepharoplasty, Reconstructive Eyelid Surgery, and Brow Lift
Medical Coverage Policy Effective Date ............................................. 4/15/2021 Next Review Date ....................................... 4/15/2022 Coverage Policy Number .................................. 0045 Blepharoplasty, Reconstructive Eyelid Surgery, and Brow Lift Table of Contents Related Coverage Resources Overview .............................................................. 1 Botulinum Therapy Coverage Policy ................................................... 1 Tissue-Engineered Skin Substitutes General Background ............................................ 3 Medicare Coverage Determinations .................... 7 Coding/Billing Information .................................... 8 References .......................................................... 9 INSTRUCTIONS FOR USE The following Coverage Policy applies to health benefit plans administered by Cigna Companies. Certain Cigna Companies and/or lines of business only provide utilization review services to clients and do not make coverage determinations. References to standard benefit plan language and coverage determinations do not apply to those clients. Coverage Policies are intended to provide guidance in interpreting certain standard benefit plans administered by Cigna Companies. Please note, the terms of a customer’s particular benefit plan document [Group Service Agreement, Evidence of Coverage, Certificate of Coverage, Summary Plan Description (SPD) or similar plan document] may differ significantly from the standard benefit plans upon which these Coverage