Development of Allergic Bronchopulmonary Aspergillosis in a Patient with Crohn's Disease
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Arthritis and Coeliac Disease
Ann Rheum Dis: first published as 10.1136/ard.44.9.592 on 1 September 1985. Downloaded from Annals of the Rheumatic Diseases 1985, 44, 592-598 Arthritis and coeliac disease J T BOURNE,' P KUMAR,2 E C HUSKISSON,' R MAGEED 3 D J UNSWORTH,3 AND J A WOJTULEWSKI4 From the Departments of 'Rheumatology and 2Gastroenterology, St Bartholomew's Hospital, West Smithfield, London ECIA 7BE; the 3Bone and Joint Research Unit, London Hospital Medical College, London El; and 4St Mary's Hospital, Eastbourne SUMMARY We report six patients with coeliac disease in whom arthritis was prominent at diagnosis and who improved with dietary therapy. Joint pain preceded diagnosis by up to three years in five patients and 15 years in one patient. Joints most commonly involved were lumbar spine, hips, and knees (four cases). In three cases there were no bowel symptoms. All were seronegative. X-rays were abnormal in two cases. HLA-type Al, B8, DR3 was present in five and B27 in two patients. Circulating immune complexes showed no consistent pattern before or after treatment. Coeliac disease was diagnosed in all patients by jejunal biopsy, and joint symptoms in all responded to a gluten-free diet. Gluten challenge (for up to three weeks) failed to provoke arthritis in three patients tested. In a separate study of 160 treated coeliac patients attending regular follow up no arthritis attributable to coeliac disease and no ankylosing was in a group spondylitis identified, though control of 100 patients with Crohn's disease thecopyright. expected incidence of seronegative polyarthritis (23%) and ankylosing spondylitis (5%) was found (p<0.01). -

Conditions Related to Inflammatory Arthritis
Conditions Related to Inflammatory Arthritis There are many conditions related to inflammatory arthritis. Some exhibit symptoms similar to those of inflammatory arthritis, some are autoimmune disorders that result from inflammatory arthritis, and some occur in conjunction with inflammatory arthritis. Related conditions are listed for information purposes only. • Adhesive capsulitis – also known as “frozen shoulder,” the connective tissue surrounding the joint becomes stiff and inflamed causing extreme pain and greatly restricting movement. • Adult onset Still’s disease – a form of arthritis characterized by high spiking fevers and a salmon- colored rash. Still’s disease is more common in children. • Caplan’s syndrome – an inflammation and scarring of the lungs in people with rheumatoid arthritis who have exposure to coal dust, as in a mine. • Celiac disease – an autoimmune disorder of the small intestine that causes malabsorption of nutrients and can eventually cause osteopenia or osteoporosis. • Dermatomyositis – a connective tissue disease characterized by inflammation of the muscles and the skin. The condition is believed to be caused either by viral infection or an autoimmune reaction. • Diabetic finger sclerosis – a complication of diabetes, causing a hardening of the skin and connective tissue in the fingers, thus causing stiffness. • Duchenne muscular dystrophy – one of the most prevalent types of muscular dystrophy, characterized by rapid muscle degeneration. • Dupuytren’s contracture – an abnormal thickening of tissues in the palm and fingers that can cause the fingers to curl. • Eosinophilic fasciitis (Shulman’s syndrome) – a condition in which the muscle tissue underneath the skin becomes swollen and thick. People with eosinophilic fasciitis have a buildup of eosinophils—a type of white blood cell—in the affected tissue. -

Hypersensitivity Reactions (Types I, II, III, IV)
Hypersensitivity Reactions (Types I, II, III, IV) April 15, 2009 Inflammatory response - local, eliminates antigen without extensively damaging the host’s tissue. Hypersensitivity - immune & inflammatory responses that are harmful to the host (von Pirquet, 1906) - Type I Produce effector molecules Capable of ingesting foreign Particles Association with parasite infection Modified from Abbas, Lichtman & Pillai, Table 19-1 Type I hypersensitivity response IgE VH V L Cε1 CL Binds to mast cell Normal serum level = 0.0003 mg/ml Binds Fc region of IgE Link Intracellular signal trans. Initiation of degranulation Larche et al. Nat. Rev. Immunol 6:761-771, 2006 Abbas, Lichtman & Pillai,19-8 Factors in the development of allergic diseases • Geographical distribution • Environmental factors - climate, air pollution, socioeconomic status • Genetic risk factors • “Hygiene hypothesis” – Older siblings, day care – Exposure to certain foods, farm animals – Exposure to antibiotics during infancy • Cytokine milieu Adapted from Bach, JF. N Engl J Med 347:911, 2002. Upham & Holt. Curr Opin Allergy Clin Immunol 5:167, 2005 Also: Papadopoulos and Kalobatsou. Curr Op Allergy Clin Immunol 7:91-95, 2007 IgE-mediated diseases in humans • Systemic (anaphylactic shock) •Asthma – Classification by immunopathological phenotype can be used to determine management strategies • Hay fever (allergic rhinitis) • Allergic conjunctivitis • Skin reactions • Food allergies Diseases in Humans (I) • Systemic anaphylaxis - potentially fatal - due to food ingestion (eggs, shellfish, -

Reactive Arthritis Information Booklet
Reactive arthritis Reactive arthritis information booklet Contents What is reactive arthritis? 4 Causes 5 Symptoms 6 Diagnosis 9 Treatment 10 Daily living 16 Diet 18 Complementary treatments 18 How will reactive arthritis affect my future? 19 Research and new developments 20 Glossary 20 We’re the 10 million people living with arthritis. We’re the carers, researchers, health professionals, friends and parents all united in Useful addresses 25 our ambition to ensure that one day, no one will have to live with Where can I find out more? 26 the pain, fatigue and isolation that arthritis causes. Talk to us 27 We understand that every day is different. We know that what works for one person may not help someone else. Our information is a collaboration of experiences, research and facts. We aim to give you everything you need to know about your condition, the treatments available and the many options you can try, so you can make the best and most informed choices for your lifestyle. We’re always happy to hear from you whether it’s with feedback on our information, to share your story, or just to find out more about the work of Versus Arthritis. Contact us at [email protected] Registered office: Versus Arthritis, Copeman House, St Mary’s Gate, Chesterfield S41 7TD Words shown in bold are explained in the glossary on p.20. Registered Charity England and Wales No. 207711, Scotland No. SC041156. Page 2 of 28 Page 3 of 28 Reactive arthritis information booklet What is reactive arthritis? However, some people find it lasts longer and can have random flare-ups years after they first get it. -

Joint Pain and Sjögren’S Syndrome
Joint Pain and Sjögren’s Syndrome Alan N. Baer, MD, FACP Alan N. Baer, MD, FACP Associate Professor of Medicine Division of Rheumatology Johns Hopkins University School of Medicine Director, Jerome Greene Sjogren's Syndrome Clinic 5200 Eastern Avenue Mason F. Lord Bldg. Center Tower Suite 4100, Room 413 Baltimore MD 21224 Phone (410) 550-1887 Fax (410) 550-6255 In 1930, Henrik Sjögren, a Swedish ophthalmologist, examined a woman with rheumatoid arthritis who had extreme dryness of her eyes and mouth and filamentary keratitis, an eye condition related to her lack of tears (1). He became fascinated by this unusual debilitating condition and subsequently evaluated 18 additional women with the same combination of findings. He described this new syndrome as “keratoconjunctivitis sicca” in his postdoctoral thesis. Thirteen of the 19 women had chronic inflammatory arthritis. We would now classify these 13 women as having secondary Sjögren’s syndrome (SS), occurring in the context of rheumatoid arthritis. However, joint pain constitutes one of the most common symptoms of the primary form of SS, defined as SS occurring in the absence of an underlying rheumatic disease. In a recent survey of SS patients belonging to the French Sjögren’s Syndrome Society (Association Française du Gougerot-Sjögren et des Syndromes Secs), 81% reported significant joint and muscle pain (2). In this article, the joint manifestations of primary SS will be reviewed. A few definitions are needed for the reader. Although the term “arthritis” was originally applied to conditions causing joint inflammation, it now includes disorders in which the joint has become damaged by degenerative, metabolic, or traumatic processes. -

A Dash of Dermatology and a Pinch of Rheumatology
A dash of dermatology and a pinch of rheumatology Sarita Nori, MD, FAAD Dermatology Atrius Health I have no conflicts of interest to disclose. 2 . Points in white = fun factoids . Points in blue = important for clinical practice; practice gaps. (Basically what you want to remember from this talk.) OUTLINE . Some clinical pearls . Treatment . Diagnosis . Update in Dermatology: . Biologics for Psoriasis - because many of your patients are on these meds now. Biologic for Atopic dermatitis – finally something new! Treatment pearls . For all itch/dermatitis: maximize anti histamines. - Zyrtec/Claritin/Allegra bid, - plus Benadryl or Hydroxyzine qhs, with intent of sedation Poison ivy - Can avoid oral steroids. Use higher topical steroids TID, + maximal anti histamines. - Poison ivy adjunct care: important to dissolve the oil from all objects that may have been exposed. Eg pets, pet collars & leashes, gardening gloves, hats, socks, shoes, watch straps, earrings etc. This is often why the rash seems to get worse despite treatment, or spreads a lot, or recurs even though it was getting better Treatment pearls . Tinea versicolor: > treat with Diflucan 400mg po x 2 doses, 1 week apart. Pityriasis Rosea: > Acyclovir 800mg 5 times /day x 1 week => 79% pts cleared by day 14. Das et al. Indian Dermatol Online J. 2015 May-Jun; 6(3): 181–184. • Seb derm on scalp/psoriasis on scalp/dandruff/itchy scalp: Ketoconazole shampoo daily, clobetasol solution qd for 2-4 weeks • Testing in allergy dept is not = skin allergy testing (patch testing). Skin contact allergies develop after multiple exposures, so not usually a new exposure. 6 MORE TREATMENT PEARLS . -

Guideline on Clinical Investigation of Medicinal Products for the Treatment of Rheumatoid Arthritis
14 December 2017 CPMP/EWP/556/95 Rev. 2 Committee for Medicinal Products for Human Use (CHMP) Guideline on clinical investigation of medicinal products for the treatment of rheumatoid arthritis Draft Agreed by Rheumatology-Immunology Working Party (RIWP) December 2011 Adoption by CHMP for release for consultation 5 December 2011 End of consultation (deadline for comments) 05 June 2012 Agreed by Rheumatology-Immunology Working Party (RIWP) March 2015 Adoption by CHMP 20 March 2015 Start of public consultation 4 June 2015 End of consultation (deadline for comments) 29 November 2015 Agreed by Rheumatology-Immunology Working Party (RIWP) 24 November 2017 Adoption by CHMP 14 December 2017 Date of coming into effect 01 July 2018 This guideline replaces the “Points to consider on the clinical investigation of medicinal products other than NSAIDS in rheumatoid arthritis (CPMP/EWP/556/95 REV. 1)” Keywords Rheumatoid arthritis, Disease Modifying Anti-Rheumatic Drugs, clinical development, CHMP, EMA, guideline 30 Churchill Place ● Canary Wharf ● London E14 5EU ● United Kingdom Telephone +44 (0)20 3660 6000 Facsimile +44 (0)20 3660 5555 Send a question via our website www.ema.europa.eu/contact An agency of the European Union © European Medicines Agency, 2018. Reproduction is authorised provided the source is acknowledged. Guideline on clinical investigation of medicinal products for the treatment of rheumatoid arthritis Table of contents List of abbreviations .................................................................................... 3 Executive -

Palindromic Rheumatism (PR), and the Drome (1)
Clinical and Experimental Rheumatology 2000; 18: 375-378. BRIEF PAPER Palindromic ABSTRACT eventually develop RA (1-3). Another Objective etiology, namely “allergic rheumatism” rheumatism: Effect of Evaluation of the contribution of dietary was suggested by Hench and Rosenberg components in triggering the attacks of in their initial description of the syn- dietary manipulation palindromic rheumatism (PR), and the drome (1). However, they could not effect of dietary manipulation on the fre- prove such an allergic origin in their G. Nesher, M. Mates quency and severity of PR attacks. cases. Methods Following a clinical observation of a Rheumatology Service, Department of Sixteen patients (10 males, 6 females) patient who experienced a complete re- Intermal Medicine, Shaare-Zedek Medical were diagnosed as having PR during mission of his PR attacks while on a low- Center, Jerusalem, Israel. 1994-8 in one center. Their mean age calorie formula preparation, it was hy- Gideon Nesher, MD, Senior Lecturer, was 45 ± 6, duration of symptoms prior pothesized that antigens in food may be Hebrew University Medical School, and to diagnosis was 4 ± 1.4 years, and fre- the triggering factors of PR attacks. Adjunct Associate Professor, St. Louis quency of PR attacks were 3.1 ± 1.8/ Thus, a prospective study was initiated University School of Medicine; Michal month. All patients were instructed to to evaluate the possible relationship be- Mates, M.D. make a list of the food that was consumed tween food and PR episodes. Please address correspondence and daily and to specify the dates of PR epi- reprint requests to: Dr. Gideon Nesher, sodes. -
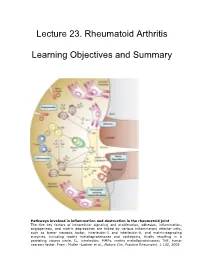
Lecture 23. Rheumatoid Arthritis Learning Objectives and Summary
Lecture 23. Rhe umatoid Arthritis Learning Objective s and Summary Pathways involved in inflammation and destruction in the rheumatoid joint The five key factors of intracellular signaling and proliferation, adhesion, inflammation, angiogenesis, and matrix degradation are linked by various inflammatory effector cells, such as tumor necrosis factor, interleukin-1 and interleukin-6, and matrix-degrading enzymes, including matrix metalloproteinases and cathepsins, finally resulting in a persisting vicious circle. IL, interleukin; MMPs, matrix metalloproteinases; TNF, tumor necrosis factor. From: Muller –Ladner et a1l. , Nature Clin. Practice Rheumatol. 1:102, 2005 23. Rheumatoid Arthritis Learning objectives: 1. To understand the clinical features of Rheumatoid Arthritis (RA) 2. To understand proposed pathophysiologic mechanisms that result in the inflammation and pathology of RA. 3. To understand the principles behind current and future therapy of RA SUMMARY 1. RA is a chronic, inflammatory autoimmune disease characterized by articular and extra-articular clinical manifestations. 2. Autoreactive antibodies in RA include rheumatoid factor and anti-CCP (cyclic citrullinated peptide) antibodies. 3. Autoreactive T cells, B cells, and an inflammatory cytokine milieu contribute to the immunopathology of RA. 4. The pannus is a membrane of granulation tissue covering the normal surface of the articular cartilages in rheumatoid arthritis. It consists of proliferating synoviocytes, infiltrating T cells, B cells, and plasma cells. Th1 cytokines are -

Rheumatoid Arthritis
RHEUMATOID ARTHRITIS Importance of IgG mediated food allergy in Rheumatoid arthritis Author: Dr. Camille Lieners INTRODUCTION FOOD AS A MAJOR TRIGGER FOR RA Rheumatoid arthritis (RA) is considered as an autoim- These facts document an ongoing systemic inflam- mune disease that leads to chronic inflammation of the matory process before the clinical manifestation of RA. joints and other parts of the body due to an overreac- The question to be asked is: What could be the cause tion of the body’s own immune system resulting in the for this inflammation? The implication of food in other attack of healthy tissues. Despite the significant impro- auto-immune diseases like celiac disease, Hashimoto vement in therapeutic management by using biologic thyroiditis, psoriasis, Lupus erythematosus removes disease-modifying drugs, true long-term remission any doubt that food may represent one of these cau- rarely occurs, and the disease progresses, leading ulti- ses, certainly not the only one, but an important one, mately to joint dysfunction and disability. as we eat every day and often this same kind of foods. (2) If this food induces an inflammatory response it will It is assumed that RA is a progressive disease starting do so continuously. Some authors also speculate it long before the appearance of symptoms. This indica- could be the fatty acids, that promote inflammation, tes the existence of a therapeutic window of oppor- but much more important are foods triggering immune tunity which is the short period of time for initiation of responses due to the presence of specific IgG antibo- efficacious therapeutic intervention, very early in the dies. -
Giant Cell Arteritis Overlapping with Rheumatoid Arthritis and Sjögren's
ogy iol : Op r B e a n l A u c c c e l e Wang et al., Mol Biol 2019, 8:2 o s s M Molecular Biology: Open Access ISSN: 2168-9547 Case Report Open Access Giant Cell Arteritis Overlapping with Rheumatoid Arthritis and Sjögren’s Syndrome Jing Wang1#, Lingyan Zhou2# and Jing Gao2* 1Department of Rheumatology, The Affiliated Hospital of Qingdao University, Qingdao, Shandong, P.R. China 2Department of Neurology, The Affiliated Hospital of Qingdao University, Qingdao, Shandong, P.R. China #These authors have contributed equally to this work Abstract Background: Giant cell arteritis (GCA) is an autoimmune vasculitis involving large and medium arteries, but the relationship with other autoimmune diseases is unclear. We reported a case of an 85-year-old woman who was diagnosed with GCA, Sjögren’s syndrome (SS) and rheumatoid arthritis (RA) simultaneously, and discussed the potential link between these diseases. Case presentation: An 85-year-old Chinese woman was admitted to hospital with aggravation of pain in two knees with headache and blurred vision. She had a history of rheumatoid arthritis and rheumatoid heart disease for 30 years and atrial fibrillation for 1 year. On admission, she had bilateral temporal pain accompanied by blurred vision. Physical examination showed dry tongue and decreased coating on the tongue. Initial laboratory results showed that C-reactive protein (CRP) was 24.89 mg/L (normally 0-5), and erythrocyte sedimentation rate (ESR) was 42.4 mm/h (normally 0-20), both of them were significantly elevated. Results: The anti-nuclear antibody (ANA) and anti-centromere antibody (ACA) was positive in serum. -

Bronchiectasis Rheumatoid Overlap Syndrome Is an Independent Risk&Nbsp
CORE Metadata, citation and similar papers at core.ac.uk Provided by AIR Universita degli studi di Milano [ Original Research Bronchiectasis ] Bronchiectasis Rheumatoid Overlap Syndrome Is an Independent Risk Factor for Mortality in Patients With Bronchiectasis A Multicenter Cohort Study Anthony De Soyza, MD, PhD; Melissa J. McDonnell, MD; Pieter C. Goeminne, MD, PhD; Stefano Aliberti, MD, PhD; Sara Lonni, MD; John Davison, RN; Lieven J. Dupont, MD, PhD; Thomas C. Fardon, MD; Robert M. Rutherford, MD; Adam T. Hill, MD; and James D. Chalmers, MD, PhD BACKGROUND: This study assessed if bronchiectasis (BR) and rheumatoid arthritis (RA), when manifesting as an overlap syndrome (BROS), were associated with worse outcomes than other BR etiologies applying the Bronchiectasis Severity Index (BSI). METHODS: Data were collected from the BSI databases of 1,716 adult patients with BR across six centers: Edinburgh, United Kingdom (608 patients); Dundee, United Kingdom (n ¼ 286); Leuven, Belgium (n ¼ 253); Monza, Italy (n ¼ 201); Galway, Ireland (n ¼ 242); and Newcastle, United Kingdom (n ¼ 126). Patients were categorized as having BROS (those with RA and BR without interstitial lung disease), idiopathic BR, bronchiectasis-COPD overlap syndrome (BCOS), and “other” BR etiologies. Mortality rates, hospitalization, and exacerbation frequency were recorded. RESULTS: A total of 147 patients with BROS (8.5% of the cohort) were identified. There was a statistically significant relationship between BROS and mortality, although this relationship was not associated with higher rates of BR exacerbations or BR-related hospitalizations. The mortality rate over a mean of 48 months was 9.3% for idiopathic BR, 8.6% in patients with other causes of BR, 18% for RA, and 28.5% for BCOS.