Plant Guide for Basalt Milkvetch (Astragalus Filipes)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(Leguminosae): Nomenclatural Proposals and New Taxa
Great Basin Naturalist Volume 58 Number 1 Article 5 1-30-1998 Astragalus (Leguminosae): nomenclatural proposals and new taxa Stanley L. Welsh Brigham Young University Follow this and additional works at: https://scholarsarchive.byu.edu/gbn Recommended Citation Welsh, Stanley L. (1998) "Astragalus (Leguminosae): nomenclatural proposals and new taxa," Great Basin Naturalist: Vol. 58 : No. 1 , Article 5. Available at: https://scholarsarchive.byu.edu/gbn/vol58/iss1/5 This Article is brought to you for free and open access by the Western North American Naturalist Publications at BYU ScholarsArchive. It has been accepted for inclusion in Great Basin Naturalist by an authorized editor of BYU ScholarsArchive. For more information, please contact [email protected], [email protected]. Great Basin Naturalist 58(1), © 1998, pp. 45-53 ASTRAGALUS (LEGUMINOSAE): NOMENCLATURAL PROPOSALS AND NEW TAXA Stanley L. Welsh! ABSTRACT.-As part of an ongoing summary revision of Astragalus for the Flora North America project, several nomenclatural changes are indicated. Nomenclatural proposals include A. molybdenus val'. shultziorom (Barneby) Welsh, comb. nov.; A. australis var. aboriginorom (Richardson) Welsh, comb. nov.; A. australis var. cattoni (M.E. Jones) Welsh, comb. nov.; A. aU8tralis var. lepagei (Hulten) Welsh, comb. nov; A. australis var. muriei (Hulten) Welsh, comb. nov.; A. subcinereus var. sileranus (M.E. Jones) Welsh, comb. nov.; A. tegetariaides val'. anxius (Meinke & Kaye) Welsh, comb. nov.; A. ampullarioides (Welsh) Welsh, comb. nov.; A. cutlen (Barneby) Welsh, comb. nov.; and A. laccaliticus (M.E. Jones) Welsh, comb. nov. Proposals of new taxa include Astragalus sect. Scytocarpi subsect. Micl'ocymbi Welsh, subsed. nov., and A. sabulosus var. -

Genetic Characterization of Three Varieties of Astragalus Lentiginosus (Fabaceae)
Genetic characterization of three varieties of Astragalus lentiginosus (Fabaceae) BRIANJ. KNAus,' RICHC. CRONN,AND AARONLISTON I Knaus, B. J. (Oregon State University, Department of Botany and Plant Pa- thology, 2082 Cordley Hall, Corvallis, OR, 97331-2902, U.S.A.; e-mail: [email protected]), R. C. Cronn (USDA Forest Service Pacific Northwest Research Station, 3200 SW Jefferson Way, Corvallis, OR, 97331, U.S .A.; e-mail: [email protected]) & A. Liston (Oregon State University, Depart- ment of Botany and Plant Pathology, 2082 Cordley Hall, Corvallis, OR, 97331- 2902, U.S.A.; e-mail: [email protected]).Genetic characterization of three varieties of Astragalus lentiginosus (Fabaceae). Brittonia 57: 334-344. 2005.-Astragalus lentiginosus is a polymorphic species that occurs in geologi- cally young habitats and whose varietal circumscription implies active morpho- logical and genetic differentiation. In this preliminary study, we evaluate the po- tential of amplified fragment length polymorphism (AFLP) markers to resolve infraspecific taxa in three varieties of Astragalus lentiginosus. Distance-based principle coordinate and neighbor-joining analyses result in clustering of individ- uals that is congruent with population origin and varietal circumscription. Anal- ysis of molecular variance of two Oregon varieties demonstrates that varietal categories account for 11% of the total variance; in contrast, geographic proximity does not contribute to the total variance. AFLPs demonstrate an ability to dis- criminate varieties of A. lentiginosus despite a potentially confounding geographic pattern, and may prove effective at inferring relationships throughout the group. Key words: AFLP, amplified fragment length polymorphism, Astragalus lenti- ginosus, genetic differentiation, infraspecific taxa. Astragalus lentiginosus Dougl. -
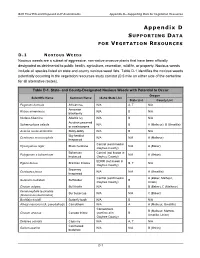
Final Environmental Impact Statement and Proposed Land-Use Plan
B2H Final EIS and Proposed LUP Amendments Appendix D—Supporting Data for Vegetation Resources Appendix D SUPPORTING DATA FOR VEGETATION RESOURCES D . 1 N O X I O U S W EEDS Noxious weeds are a subset of aggressive, non-native invasive plants that have been officially designated as detrimental to public health, agriculture, recreation, wildlife, or property. Noxious weeds include all species listed on state and county noxious weed lists. Table D-1 identifies the noxious weeds potentially occurring in the vegetation resources study corridor (0.5 mile on either side of the centerline for all alternative routes). Table D-1. State- and County-Designated Noxious Weeds with Potential to Occur Oregon Scientific Name Common Name Idaho State List State List County List Peganum harmala African rue N/A A, T N/A Armenian Rubus armeniacus N/A B N/A blackberry Hedera hibernica Atlantic Ivy N/A B N/A Austrian peaweed Sphaerophysa salsula N/A B A (Malheur), B (Umatilla) or swainsonpea Acaena novae-zelandiae Biddy-biddy N/A B N/A Big-headed Centaurea macrocephala N/A N/A A (Malheur) knapweed Control (confirmed in Hyoscyamus niger Black henbane N/A A (Baker) Owyhee County) Bohemian Control (not known in Polygonum x bohemicum N/A A (Union) knotweed Owyhee County) EDRR (not known in Egeria densa Brazilian Elodea B, T N/A Owyhee County) Brownray Centaurea jacea N/A N/A A (Umatilla) knapweed Control (confirmed in A (Baker, Malheur, Solanum rostratum Buffalobur B Owyhee County) Union) Cirsium vulgare Bull thistle N/A B B (Baker), C (Malheur) Ceratocephala testiculata Bur buttercup N/A N/A C (Baker) (Ranunculus testiculatus) Buddleja davidii Butterfly bush N/A B N/A Alhagi maurorum (A. -

Too Wild to Drill A
TOO WILD TO DRILL A The connection between people and nature runs deep, and the sights, sounds and smells of the great outdoors instantly remind us of how strong that connection is. Whether we’re laughing with our kids at the local fishing hole, hiking or hunting in the backcountry, or taking in the view at a scenic overlook, we all share a sense of wonder about what the natural world has to offer us. Americans around the nation are blessed with incredible wildlands out our back doors. The health of our public lands and wild places is directly tied to the health of our families, communities and economy. Unfortunately, our public lands and clean air and water are under attack. The Trump administration and some in Congress harbor deep ties to fossil fuel and mining interests, and today, resource extraction lobbyists see an unprecedented opportunity to open vast swaths of our public lands. Recent proposals to open the Arctic National Wildlife Refuge to drilling and shrink or eliminate protected lands around the country underscore how serious this threat is. Though some places are appropriate for responsible energy development, the current agenda in Washington, D.C. to aggressively prioritize oil, gas and coal production at the expense of all else threatens to push drilling and mining deeper into our wildest forests, deserts and grasslands. Places where families camp and hike today could soon be covered with mazes of pipelines, drill rigs and heavy machinery, or contaminated with leaks and spills. This report highlights 15 American places that are simply too important, too special, too valuable to be destroyed for short-lived commercial gains. -

Owyhee Desert Sagebrush Focal Area Fuel Breaks
B L M U.S. Department of the Interior Bureau of Land Management Decision Record - Memorandum Owyhee Desert Sagebrush Focal Area Fuel Breaks PREPARING OFFICE U.S. Department of the Interior Bureau of Land Management 3900 E. Idaho St. Elko, NV 89801 Decision Record - Memorandum Owyhee Desert Sagebrush Focal Area Fuel Breaks Prepared by U.S. Department of the Interior Bureau of Land Management Elko, NV This page intentionally left blank Decision Record - Memorandum iii Table of Contents _1. Owyhee Desert Sagebrush Focal Area Fuel Breaks Decision Record Memorandum ....... 1 _1.1. Proposed Decision .......................................................................................................... 1 _1.2. Compliance ..................................................................................................................... 6 _1.3. Public Involvement ......................................................................................................... 7 _1.4. Rationale ......................................................................................................................... 7 _1.5. Authority ......................................................................................................................... 8 _1.6. Provisions for Protest, Appeal, and Petition for Stay ..................................................... 9 _1.7. Authorized Officer .......................................................................................................... 9 _1.8. Contact Person ............................................................................................................... -

Growth Curve of White-Tailed Antelope Squirrels from Idaho
Western Wildlife 6:18–20 • 2019 Submitted: 24 February 2019; Accepted: 3 May 2019. GROWTH CURVE OF WHITE-TAILED ANTELOPE SQUIRRELS FROM IDAHO ROBERTO REFINETTI Department of Psychological Science, Boise State University, Boise, Idaho 83725, email: [email protected] Abstract.—Daytime rodent trapping in the Owyhee Desert of Idaho produced a single diurnal species: the White-tailed Antelope Squirrel (Ammospermophilus leucurus). I found four females that were pregnant and took them back to my laboratory to give birth and I raised their litters in captivity. Litter size ranged from 10 to 12 pups. The pups were born weighing 3–4 g, with purple skin color and with the eyes closed. Pups were successfully weaned at 60 d of age and approached the adult body mass of 124 g at 4 mo of age. Key Words.—Ammospermophilus leucurus; Great Basin Desert; growth; Idaho; Owyhee County The White-tailed Antelope Squirrel (Ammo- not differ significantly (t = 0.715, df = 10, P = 0.503). spermophilus leucurus; Fig. 1) is indigenous to a large After four months in the laboratory (after parturition and segment of western North America, from as far north lactation for the four pregnant females), average body as southern Idaho and Oregon (43° N) to as far south mass stabilized at 124 g (106–145 g). as the tip of the Baja California peninsula (23° N; Belk The four pregnant females were left undisturbed and Smith 1991; Koprowski et al. 2016). I surveyed a in individual polypropylene cages with wire tops (36 small part of the northernmost extension of the range cm length, 24 cm width, 19 cm height). -

New Combination in Astragalus (Fabaceae)
Smith, J.F. and J.C. Zimmers. 2017. New combination in Astragalus (Fabaceae). Phytoneuron 2017-38: 1–3. Published 1 June 2017. ISSN 2153 733X NEW COMBINATION IN ASTRAGALUS (FABACEAE) JAMES F. SMITH and JAY C. ZIMMERS Department of Biological Sciences Snake River Plains Herbarium Boise State University Boise, Idaho 83725 [email protected] ABSTRACT Recent molecular phylogenetic analyses have established that the four varieties of Astragalus cusickii are three distinct, monophyletic clades: A. cusickii var. cusickii and A. cusickii var. flexilipes form one clade, A. cusickii var. sterilis and A. cusickii var. packardiae each form the other two. Although relationships among the clades in the analyses are poorly resolved, they are also poorly resolved with respect to other recognized species in the genus. Morphological data provide unique synapomorphies for each of the clades and therefore we propose to recognize three distinct species, with A. cusickii var. flexilipes retained at the rank of variety. A new combination brings A. cusickii var. packardiae to species rank, as Astragalus packardiae (Barneby) J.F. Sm. & Zimmers, comb. nov. , whereas A. sterilis has already been published. Astragalus L. is a diverse group of approximately 2500 species (Frodin 2004; Lock & Schrire 2005; Mabberley 2008) and has a rich diversity in four geographic areas (southwest and south-central Asia, the Sino-Himalayan region, the Mediterranean Basin, and western North America; in addition the Andes in South America have at least 100 species. Second to Eurasia in terms of species diversity is the New World, with approximately 400-450 species. The Intermountain Region of western North America (Barneby 1989) is especially diverse, and an estimated 70 species of Astragalus can be found in Idaho alone, including several endemic taxa (Mancuso 1999). -

Nutritional and Functional Characteristics of Erophaca Baetica Seeds, a Legume Endemic to the Mediterranean Region
GRASAS Y ACEITES, 64 (3), ABRIL-JUNIO, 229-236, 2013, ISSN: 0017-3495 DOI: 10.3989/gya.107412 INVESTIGACIÓNINVESTIGACIÓN Nutritional and functional characteristics of Erophaca baetica seeds, a legume endemic to the Mediterranean region By I. Cortés-Giraldo, M. Alaiz, J. Girón-Calle, C. Megías and J. Vioque* Instituto de la Grasa (C.S.I.C.). Avda. Padre García Tejero 4. 41012 Sevilla, Spain * Corresponding author: [email protected] RESUMEN and linoleic acids are the major fatty acids in the seeds. The antioxidant activity of polyphenol extracts was higher than the Características nutricionales y funcionales de semi- activity of the polyphenols extracted from most edible legume llas de Erophaca baetica, una leguminosa endémica de seeds. Hence, E. baetica seeds represent a promising sour- la región Mediterránea ce of functional and nutritional components on the condition that the anti-nutritional alkaloids are previously removed. Erophaca baetica es una leguminosa endémica de la re- gión mediterránea. Aunque los frutos y las semillas son gran- KEY-WORDS: Amino acids – Erophaca baetica – Fatty des, la presencia del alcaloide swainsonina causante de locois- acids – Polyphenols – Protein. mo, ha impedido su uso como alimento para animales o para nutrición humana. El contenido proteico y su perfil cromatográ- fico, la composición de aminoácidos, de ácidos grasos, y con- 1. INTRODUCTION tenido de polifenoles se han determinado con el fin de explorar el potencial de las semillas de E. baetica como una fuente de Legumes are of nutritional interest not only proteínas y de componentes funcionales de la dieta. El conteni- because of their high quality protein, but also do de proteínas es del 36% (w / w), y el análisis de aminoáci- dos reveló deficiencia en aminoácidos azufrados, triptófano y because they are rich in functional components lisina. -

Mf2612 Chinese Milkvetch
Research and Extension: MF-2612 A Grower’s Guide Chinese Milkvetch Astragalus membranaceus This plant is widely and safely used in Chinese medicine but is related to many species from North America, including Missouri milkvetch (A. missouriensis) and woolly loco (A. mollissimus), which are poisonous to livestock. Family: Fabaceae generally between the third and fifth year perennial flower bed or as a field crop. Life cycle: Herbaceous perennial depending on location and how fast the Though we had high hopes for this crop, (Zone 5) plants grow. Dig roots using a needle-nose the root yields in year three were not spade or a garden fork to extract the large. Potential demand is still high Native: Northeastern China entire root. Appears to be a taproot with because this is a widely used herb with Height: 3 to 4 feet, sprawls as it matures branches. Harvest could be partially many properties. Digging and drying the mechanized. root can be a lot of work, but mechaniza- Sun: Partial shade to full sun tion may be possible. The plant does not Parts used: Roots, fresh or dried. Soil: Well worked, sandy, dry soil have many insect or disease pests, but Used as: Medicinal food, tonic, decoc- likes well-drained soil. It needs a bit of Water: Moderate, will not do well in tion, traditional tincture, syrup, elixir, coddling for the first couple of months poorly drained soil lozenge, honey, powder after transplanting because it grows slowly Flowers: Pale yellow, blooms from mid- Medicinal benefits: Stimulates the the first year. It may not work as a direct- summer until frost immune system. -

BWSR Featured Plant Statewide Wetland Name: Prairie Milk Vetch (Astragalus Adsurgens) Indicator Status: Also Called: Standing Milk-Vetch, Lavender Milk-Vetch UPL
BWSR Featured Plant Statewide Wetland Name: Prairie Milk Vetch (Astragalus adsurgens) Indicator Status: Also called: Standing Milk-vetch, Lavender Milk-vetch UPL Plant Family: Fabaceae (Pea) Prairie Milk Vetch is a flowering perrenial herb of the pea (legume or bean) family, one of the three largest families of terrestrial plants. A native plant to Minnesota, it is found mostly on the western half of the state. It blooms in June and July where it prefers full sun in open grasslands and dry prairies. Identification Plants are typically around one-foot tall but can grow up to sixteen inches. The plants have many stems and can spread up to two feet across from a center crown. The stems can vary from being Prairie Milk Vetch Image by Peter M. Dziuk of Minnesota erect to lying on the ground. Compound leaves Wildflowers are attached alternately in groups of thirteen to twenty-one. Each leaf is about three inches long with individual leaflets up to one inch long. The lavender to bluish flowers are grouped in round clusters about one inch wide and up to two inches tall. Each flower is held by a light green calyx that will form a hairy pod (legume). Range Prairie Milk Vetch is found in the U.S. from Washington to Minnesota and as far south as New Mexico, where it can be found at elevations up to 11,000 feet. It is found in every province in Canada. A variety of A. adsurgens called Standing Milk-vetch is found in China and Leaves and stems of Prairie Milk Vetch Image by Peter M. -

Sendtnera 1: 267-272
© Biodiversity Heritage Library, http://www.biodiversitylibrary.org/; www.biologiezentrum.at 267 Beiträge zur Kenntnis der Gattung Astragalus L. (Leguminosae) III- VI von D. PODLECH Abstract: PODLECH, D.: Beiträge zur Kenntnis der Gattung Astragalus L. (Leguminosae) III-VI. Sendtnera 1: 267-272. 1993. ISSN 0944-0178. ni: The systematic position of Astragalus lusitanicus Lam. as a member of the tribe Sophoreae is discussed. - IV: New names and combinations within the genera Astragalus L. and Astracantha Podlech are introduced. - V: The taxonomic position of some hitherto not recognised names of LINNE is discussed. - VI. Astragalus coluteocarpus Boiss. subsp. heratensis Podlech is described. m. Was ist Astragalus lusitanicus Lam. ? Astragalus lusitanicus Lam. ist eine große, stattliche Pflanze, die im westlichen Mittelmeer- gebiet schon früh Beachtung gefunden hat. LiNNE beschrieb sie als Phaca baetica, Lamarck mußte bei der Überfuhrung in die Gattung einen neuen Namen wählen, da Astragalus boeticus L. bereits vergeben war. Innerhalb der Gattung Astragalus war unsere Art vor allem wegen der großen, aufgeblasenen, unilokulären Hülsen und wegen des schiefen glockigen Kelches immer ein Fremdkörper, was BOISSIER, den hervorragenden Kenner der Gattung veranlaßte, für sie eine eigene Gattung Erophaca zu bilden. Dennoch galt die Art bis heute unangefochten als eine Vertreterin der Gattung Astragalus. Eine genaue Merkmalsanalyse ergab jedoch eine Reihe auffallend abweichender Merkmale, so daß die Zugehörigkeit zur Gattung Astragalus nicht länger aufrecht erhalten werden kann. Seitlich angeheftete und schwach gehöckerte Kelche mit schief abgeschnittener ÖflBiung finden sich zwar in einigen basalen Gruppen der Gattung sowie in deren Verwandtschaft bei Chesneya und Gueldenstaedtia, jedoch verbietet dieses Merkmal in Verbindung mit anderen, bisher übersehenen Charakteren eindeutig einen Verbleib in der Tribus der Galegeae, zu der Astragalus und Verwandte zu rechnen sind. -

Reproductive Ecology of Astragalus Filipes, a Great Basin
REPRODUCTIVE ECOLOGY OF ASTRAGALUS FILIPES, A GREAT BASIN RESTORATION LEGUME by Kristal M. Watrous A thesis submitted in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE in Biology Approved: ________________________ _______________________ James H. Cane Edward W. Evans Major Professor Committee Member ________________________ _______________________ Eugene W. Schupp Byron R. Burnham Committee Member Dean of Graduate Studies UTAH STATE UNIVERSITY Logan, Utah 2010 ii ABSTRACT Reproductive Ecology of Astragalus filipes, a Great Basin Restoration Legume by Kristal M. Watrous, Master of Science Utah State University, 2010 Major Professor: Dr. James H. Cane Department: Biology Astragalus filipes Torrey ex. A. Gray (Fabaceae) is being studied and propagated for use in rangeland restoration projects throughout the Great Basin. Restoration forbs often require sufficient pollination services for seed production and persistence in restoration sites. Knowledge of a plant’s breeding biology is important in providing pollination for maximal seed set. Reproductive output from four manual pollination treatments (autogamy, geitonogamy, xenogamy, and distant xenogamy) was examined in a common garden. Pod set, seed set, and seed germination were quantified for each of the treatments. Seed set from four wild populations was compared to that of an openly visited common garden array. A. filipes was found to be self-compatible, but to benefit greatly from outcrossing. Less seed germinated from distantly outcrossed treatments than for any other treatment, indicating possible outbreeding depression. Common garden plants set less seed per pod than any wild population, possibly due to a depauperate pollinator guild in the common garden. iii Bees were surveyed at wild A. filipes populations to identify common pollinators.