Catchment-Scale Ecological Risk Assessment of Pesticides
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Onesided Livebearer (Jenynsia Lineata)
Onesided Livebearer (Jenynsia lineata; a fish) Ecological Risk Screening Summary U.S. Fish and Wildlife Service, July 2017 Revised, January 2018 Web Version, 8/16/2018 1 Native Range and Status in the United States Native Range From Froese and Pauly (2017): “South America: southern tributaries of the Mirim Lagoon.” Froese and Pauly (2017) list this species as native to Argentina (Cordiviola de Yuan and Pignalberi de Hassan 1985), Brazil (Wischnath 1993), and Uruguay (Ghedotti 1998). Status in the United States No known occurrences in the United States. From Hellweg (2014): “Their cousins the Jenynsia are sometimes available in hobbyist circles. There are a dozen or so species that have been popularized as the one-sided livebearer.” 1 Means of Introductions in the United States No known occurrences in the United States. Remarks From Froese and Pauly (2017): “Synonym: Lebias lineata. CoL [Catalogue of Life] Status: synonym. Synonym: Fitzroyia lineata. CoL Status: synonym.” Both the above synonyms were used in addition to the accepted scientific name to search for information about this species. 2 Biology and Ecology Taxonomic Hierarchy and Taxonomic Standing From ITIS (2017): “Kingdom Animalia Infrakingdom Deuterostomia Phylum Chordata Subphylum Vertebrata Infraphylum Gnathostomata Superclass Actinopterygii Class Teleostei Superorder Acanthopterygii Order Cyprinodontiformes Suborder Cyprinodontoidei Family Anablepidae Subfamily Anablepinae Genus Jenynsia Species Jenynsia lineata (Jenyns 1842)” “Taxonomic Status: valid” Size, Weight, and -

Reproductive Aspects of the One-Sided Livebearer Jenynsia Multidentata (Jenyns, 1842) (Cyprinodontiformes) in the Patos Lagoon Estuary, Brazil
Reproductive aspects of the one-sided livebearer Jenynsia multidentata (Jenyns, 1842) (Cyprinodontiformes) in the Patos Lagoon estuary, Brazil 1 2 2 3 ANA C. G. MAI , ALEXANDRE M. GARCIA , JOÃO P. VIEIRA & MÔNICA G. MAI 1 Programa de Pós-graduação em Ciências Biológicas (Zoologia). Universidade Federal da Paraíba. Departamento de Sistemática e Ecologia. Centro de Ciências Exatas e da Natureza, LAPEC. CEP 58059-900, João Pessoa, Paraíba, Brasil. E-mail: [email protected] 2 Fundação Universidade Federal do Rio Grande, Departamento de Oceanografia, Laboratório de Ictiologia, Caixa Postal 474, Rio Grande, Rio Grande do Sul, Brasil. [email protected] ou [email protected] 3 Universidade Federal de São Carlos. Centro de Ciências Biológicas e da Saúde, Departamento de Hidrobiologia, Caixa Postal: 676, São Carlos, São Paulo, Brasil. [email protected] Abstract. Jenynsia multidentata (Jenyns, 1842) is a viviparous fish found year-round in the estuary zone of Patos Lagoon and represents one of the dominant species in its shallow areas. Although biological and ecological aspects of the J. multidentata's population occurring in Patos Lagoon estuary have been previously investigated, there is no published information regarding its reproductive biology in this estuarine area. The reproductive cycle of this species in the estuarine zone of Patos Lagoon occurred from September to May, showing a positive correlation with water temperature (r = 0.76). The sexual ratio in the estuary was 1.98/1 (female/male) and female size and fecundity had a positive correlation. It was observed that J. multidentata presented a second pregnancy during January and February months, which has not been described in previous studies. -

Estructura, Composición Y Dinámica Estacional De Las Comunidades De Peces De Las Lagunas Encadenadas Del Oeste
UNIVERSIDAD NACIONAL DEL SUR TESIS DE DOCTORADO EN CIENCIAS BIOLÓGICAS ESTRUCTURA, COMPOSICIÓN Y DINÁMICA ESTACIONAL DE LAS COMUNIDADES DE PECES DEL SISTEMA DE LAS LAGUNAS ENCADENADAS DEL OESTE, PROVINCIA DE BUENOS AIRES. LICENCIADO MARCELO GABRIEL SCHWERDT DIRECTORA: DOCTORA ANDREA LOPEZ CAZORLA BAHÍA BLANCA ARGENTINA 2012 Estructura, composición y dinámica estacional de las comunidades ii de peces de las lagunas Encadenadas del Oeste DECLARACIÓN Esta tesis ha sido presentada como parte de los requisitos para optar al grado Académico de Doctor en Ciencias Biológicas, de la Universidad Nacional del Sur. Declaro que el material incluido en esta tesis es, a mi mejor saber y entender, original, producto de mi propio trabajo (salvo en la medida que se identifique explícitamente las contribuciones de otros), y que este material no lo he presentado, en forma parcial o total, como una tesis en ésta u otra institución. La misma contiene los resultados obtenidos en investigaciones llevadas a cabo en el Departamento de Biología, Bioquímica y Farmacia de la Universidad Nacional del Sur, durante el período comprendido entre el 1 de abril de 2006 y el 1 de abril de 2012, bajo la dirección de la Dra. Andrea Lopez Cazorla, Profesora Adjunta de la Cátedra de Vertebrados. Estructura, composición y dinámica estacional de las comunidades iii de peces de las lagunas Encadenadas del Oeste DEDICATORIA Para mi amor y compañera de la vida, Jorgelina y nuestro hijo Alejo, alma y motor motivador de todo proyecto e iniciativa que emprendo. Gracias por inspirarme, día tras día. Estructura, composición y dinámica estacional de las comunidades iv de peces de las lagunas Encadenadas del Oeste AGRADECIMIENTOS Al Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) por decidir que el proyecto debía ser financiado y apoyado, permitiéndome así realizar las tareas de investigación que forman parte de la presente tesis y concretar mis estudios de posgrado. -

Reproductive Cycle and Spatiotemporal Variation in Abundance of the One-Sided Livebearer Jenynsia Multidentata, in Patos Lagoon, Brazil
Hydrobiologia 515: 39–48, 2004. 39 © 2004 Kluwer Academic Publishers. Printed in the Netherlands. Reproductive cycle and spatiotemporal variation in abundance of the one-sided livebearer Jenynsia multidentata, in Patos Lagoon, Brazil Alexandre M. Garcia1, João P. Vieira1, Kirk O. Winemiller2 & Marcelo B. Raseira1 1Fundação Universidade Federal de Rio Grande, Departamento de Oceanografia, Laborat´orio de Ictiologia, C. P. 474, Rio Grande, RS, Brazil E-mail: [email protected] 2Department of Wildlife and Fisheries Sciences, Texas A&M University, College Station, TX 77843-2258, U.S.A. Received 17 June 2003; in revised form 25 August 2003; accepted 27 August 2003 Key words: Anablepidae, life-history, livebearing fishes, Patos Lagoon estuary Abstract Jenynsia multidentata is an important component of the fish assemblage of the Patos Lagoon estuary in southern South Brazil. In order to investigate its reproductive cycle and abundance patterns, standardized sampling was conducted over large spatial (marine, estuary and lagoon) and temporal (1996–2003) scales. Both females and males were significantly more abundant during summer (December–March) than winter (June–August). Total abundance was significantly positively correlated with water temperature (R = 0.91), but not with salinity and Secchi depth. Females achieved higher average (49.1 mm LT) and maximum size (91 mm) than males (37.7 mm; 66 mm), and average sex ratio was female-biased (3.2:1) across all months. An annual reproductive cycle composed of two cohorts was proposed: individuals born from December to March started reproducing during late winter and spring and individuals born from September to November started reproducing during late summer and fall. -

Download Full Issue
Pan-American Journal of Aquatic Sciences 2(1) 2007 This volume of our Journal is humbly dedicated to the memory of our colleague and friend Lic. Guillermo Armando Verazay (1953-2007), a researcher from INIDEP, Argentina, and also Dr. Ransom Myers (1952-2007), from Dalhousie University, Canada, whose work inspires us. Pan-American Journal of Aquatic Sciences Research articles Limpeza de ambientes costeiros brasileiros contaminados por petróleo: uma revisão. CANTAGALLO, C., MILANELLI, J. C. C & DIAS-BRITO, D…..................................................................1 Short-term changes in the concentration and vertical distribution of chlorophyll and in the structure of the microplankton assemblage due to a storm CALLIARI, D………………………………………………….……………………………………...13 Reversal and ambicoloration in two flounder species (Paralichthyidae, Pleuronectiformes). LUIZ CONSTANTINO DA SILVA JUNIOR, AMANDA C. DE ANDRADE, MAGDA F. DE ANDRADE-TUBINO, VIANNA, M..........................................................................................................................................23 Utilization patterns of surf zone inhabiting fish from beaches in Southern Brazil. FÉLIX, F. C. SPACH, H. L., MORO, P. S., SCHWARZ JR., R., SANTOS, C., HACKRADT, C. W. & HOSTIM- SILVA, M.............................................................................................................................................27 Reproductive aspects of the one-sided livebearer Jenynsia multidentata (Jenyns, 1842) (Cyprinodontiformes) in the Patos Lagoon -

Trichodinids (Ciliophora) of Corydoras Paleatus (Siluriformes) And
Acta Protozool. (2016) 55: 249–257 www.ejournals.eu/Acta-Protozoologica ACTA doi:10.4467/16890027AP.16.027.6096 PROTOZOOLOGICA Trichodinids (Ciliophora) of Corydoras paleatus (Siluriformes) and Jenynsia multidentata (Cyprinodontiformes) from Argentina, with Description of Trichodina corydori n. sp. and Trichodina jenynsii n. sp. Paula S. MARCOTEGUI1, Linda BASSON2, and Sergio R. MARTORELLI1 1 Centro de Estudios Parasitológicos y de Vectores (CEPAVE) (CCT-La Plata-CONICET-UNLP), Argentina; 2 Department of Zoology and Entomology, University of the Free State, Bloemfontein, Republic of South Africa Abstract. During surveys of parasites of the pepper cory Corydoras paleatus Jenyns, 1842 and sided-livebearer Jenynsia multidentata Jenyns, 1842 from Samborombón River, Argentina, Trichodina corydori n. sp., Trichodina cribbi Dove and O’Donoghue, 2005 and T. je- nynsii n. sp. were morphologically studied. Taxonomic and morphometric data for these trichodinids based on dry silver nitrate-impregnated specimens are presented. Trichodina corydori is characterized by a prominent blade apophysis, the section connecting the blade and central part is short, and the adoral ciliary spiral makes a turn of 370–380°. Trichodina jenynsii is characterized by curved blades and prominently- shaped denticle rays that are characteristically extremely long, tapering to thin sharp points in adult specimens. This study is the first formal report of these trichodinids from South America, and the description of two new species. Key words: Ciliophora, Trichodina, fish parasites, gill parasites, trichodinids, Argentina. INTRODUCTION mccradyi (Mayer) (Martorelli et al. 2008). Marcotegui and Martorelli (2009) described Trichodina scalensis Marcotegui et Martorelli, 2009 from Mugil liza (Va- Trichodinid ciliophorans have rarely been studied in lenciennes), and reported Trichodina puytoraci Lom, South America (Martins and Ghiraldelli 2008, Miranda 1962, T. -
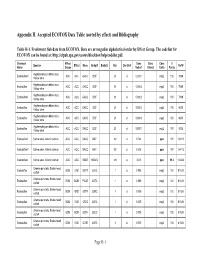
Appendix H. Accepted ECOTOX Data Table (Sorted by Effect) and Bibliography
Appendix H. Accepted ECOTOX Data Table (sorted by effect) and Bibliography Table H-1. Freshwater fish data from ECOTOX. Data are arranged in alphabetical order by Effect Group. The code list for ECOTOX can be found at: http://cfpub.epa.gov/ecotox/blackbox/help/codelist.pdf. Chemical Effect Conc Conc Conc % Species Effect Meas Endpt1 Endpt2 Dur Dur Unit Ref # Name Group Value1 Value2 Units Purity Hyphessobrycon bifasciatus, Endosulfan II ACC ACC GACC BCF 21 d 0.0001 mg/L 100 7009 Yellow tetra Hyphessobrycon bifasciatus, Endosulfan ACC ACC GACC BCF 21 d 0.0003 mg/L 100 7009 Yellow tetra Hyphessobrycon bifasciatus, Endosulfan I ACC ACC GACC BCF 21 d 0.0002 mg/L 100 7009 Yellow tetra Hyphessobrycon bifasciatus, Endosulfan ACC ACC GACC BCF 21 d 0.0002 mg/L 100 8035 Yellow tetra Hyphessobrycon bifasciatus, Endosulfan ACC ACC GACC BCF 21 d 0.0003 mg/L 100 8035 Yellow tetra Hyphessobrycon bifasciatus, Endosulfan ACC ACC GACC BCF 21 d 0.0001 mg/L 100 8035 Yellow tetra Endosulfan I Salmo salar, Atlantic salmon ACC ACC GACC BAF 92 d 0.724 ppm 100 104113 Endosulfan II Salmo salar, Atlantic salmon ACC ACC GACC BAF 92 d 0.315 ppm 100 104113 Endosulfan I Salmo salar, Atlantic salmon ACC ACC RSDE NOAEL 49 d 0.03 ppm 99.8 104340 Channa punctata, Snake-head Endosulfan BCM ENZ GSTR LOEC 1 d 0.005 mg/L 100 81028 catfish Channa punctata, Snake-head Endosulfan BCM BCM PCAR LOEC 1 d 0.005 mg/L 100 81028 catfish Channa punctata, Snake-head Endosulfan BCM ENZ GSTR LOEC 1 d 0.005 mg/L 100 81028 catfish Channa punctata, Snake-head Endosulfan BCM ENZ CTLS LOEC 1 d 0.005 mg/L 100 81028 catfish Channa punctata, Snake-head Endosulfan BCM BCM GLTH LOEC 1 d 0.005 mg/L 100 81028 catfish Channa punctata, Snake-head Endosulfan BCM ENZ GLRE LOEC 1 d 0.005 mg/L 100 81028 catfish Page H-1 Table H-1. -

Appendix J. Aquatic Ecological Effects Data
Appendix J. Aquatic Ecological Effects Data This appendix is a summary of the data being evaluated for ecological effects on aquatic organisms. Each section consists of several sets of data. Tables presenting the data submitted with the application for registration are presented first. The lowest value from this table is used to set a “benchmark” that is used in evaluating the data from the open literature—from EPA’s ECOTOX database. Only the studies associated with the ECOTOX data that have values less than these benchmarks are examined further. If any of these latter values are acceptable, then that value becomes the final value used in the risk assessment for that particular taxonomic group. Because the stability of endosulfan TGAI in aquatic tests is a concern due to its volatility, preference was given to those studies that quantified endosulfan exposure via analytical measurements and where appropriate, used a flow-through exposure regime. In some cases, however, these data were lacking (e.g., aquatic nonvascular plants), and toxicity values based on nominal concentrations were used to fulfill a data gap. In general, studies from the open literature do not provide sufficient documentation for evaluating all or most of the OPPTS Guideline evaluation criteria. Consistent with the Overview Document (USEPA 2004), if sufficient documentation was provided to judge that the open literature studies were scientifically valid and acceptable endpoints were determined that were below the ‘benchmark’ values, they were typically classified as supplemental-- but acceptable for quantitative use. If sufficient documentation was not provided to comprehensively judge the scientific validity of the study but no information existed that suggested the study would be invalid, these studies were typically classified as supplemental--but acceptable for qualitative use. -
Evolution of Vertebrate Vision by Means of Whole Genome Duplications
Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Medicine 1070 Evolution of Vertebrate Vision by Means of Whole Genome Duplications Zebrafish as a Model for Gene Specialisation DAVID LAGMAN ACTA UNIVERSITATIS UPSALIENSIS ISSN 1651-6206 ISBN 978-91-554-9155-0 UPPSALA urn:nbn:se:uu:diva-242781 2015 Dissertation presented at Uppsala University to be publicly examined in B:41, Biomedicinskt centrum, Husargatan 3, Uppsala, Friday, 20 March 2015 at 09:15 for the degree of Doctor of Philosophy (Faculty of Medicine). The examination will be conducted in English. Faculty examiner: Professor Stephan Neuhauss ( University of Zurich, Institute of Molecular Life Sciences, Zurich, Switzerland ). Abstract Lagman, D. 2015. Evolution of Vertebrate Vision by Means of Whole Genome Duplications. Zebrafish as a Model for Gene Specialisation. Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Medicine 1070. 57 pp. Uppsala: Acta Universitatis Upsaliensis. ISBN 978-91-554-9155-0. The signalling cascade of rods and cones use different but related protein components. Rods and cones, emerged in the common ancestor of vertebrates around 500 million years ago around when two whole genome duplications took place, named 1R and 2R. These generated a large number of additional genes that could evolve new or more specialised functions. A third event, 3R, occurred in the ancestor of teleost fish. This thesis describes extensive phylogenetic and comparative synteny analyses of the opsins, transducin and phosphodiesterase (PDE6) of this cascade by including data from a wide selection of vertebrates. The expression of the zebrafish genes was also investigated. The results show that genes for these proteins duplicated in 1R and 2R as well as some in 3R. -
A Macroscopic Classification of the Embryonic Development of the One-Sided Livebearer Jenynsia Multidentata (Teleostei: Anablepidae)
Neotropical Ichthyology, 15(4): e160170, 2017 Journal homepage: www.scielo.br/ni DOI: 10.1590/1982-0224-20160170 Published online: 18 December 2017 (ISSN 1982-0224) Copyright © 2017 Sociedade Brasileira de Ictiologia Printed: 18 December 2017 (ISSN 1679-6225) A macroscopic classification of the embryonic development of the one-sided livebearer Jenynsia multidentata (Teleostei: Anablepidae) Nathalia C. López-Rodríguez1, Cíntia M. de Barros2 and Ana Cristina Petry2 This study proposes eight stages according to the main discernible changes recorded throughout the embryonic development of Jenynsia multidentata. The development of morphological embryo structures, pigmentation, and changes in tissues connecting mother and embryo were included in the stage characterization. From the fertilized egg (Stage 1), an embryo reaches the intermediary stages when presenting yolk syncytial layer (Stage 2), initial pigmentation of the outer layers of the retina and dorsal region of the head (Stage 3), and the sprouting of the caudal (Stage 4), dorsal and anal fins (Stage 5). During the later stages, the ovarian folds enter the gills, and the body pigmentation becomes more intense (Stage 6), the body becomes elongated (Stage 7), and there is a greater intensity in body pigmentation and increased muscle mass (Stage 8). The dry weight of the batches varied between 0.6 ± 0.3 mg (Stage 3) to 54.6 ± 19.7 mg (Stage 8), but the dry weight of the maternal-embryonic connecting tissues remained almost constant. After controlling the effect of those reproductive tissues, the gain in dry weight of the batches throughout development increased exponentially from Stage 6, reflecting the increase in size and weight of the embryos due to matrotrophy. -
Divergence in the Ecology of Two Species of Gambusia in Secondary Contact Daniella M
University of New Mexico UNM Digital Repository Biology ETDs Electronic Theses and Dissertations 5-1-2011 Divergence in the ecology of two species of Gambusia in secondary contact Daniella M. Swenton Follow this and additional works at: https://digitalrepository.unm.edu/biol_etds Recommended Citation Swenton, Daniella M.. "Divergence in the ecology of two species of Gambusia in secondary contact." (2011). https://digitalrepository.unm.edu/biol_etds/105 This Dissertation is brought to you for free and open access by the Electronic Theses and Dissertations at UNM Digital Repository. It has been accepted for inclusion in Biology ETDs by an authorized administrator of UNM Digital Repository. For more information, please contact [email protected]. DIVERGENCE IN THE ECOLOGY OF TWO SPECIES OF GAMBUSIA IN SECONDARY CONTACT BY Daniella M. Swenton B.S., Environmental Science, University of Vermont, 2003 DISSERTATION Submitted in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy Biology The University of New Mexico Albuquerque, New Mexico May, 2011 Dedication This dissertation is dedicated to my loved ones. My dad adores exploring nature and inspired that passion in me. Deb is a well of unconditional love & support. My sister, Deborah, & Hank, Bean, Jas, & Mer are the calm anchor to my whipping kite. My mom always encourages me to reach higher. My brothers keep me on my toes and in smiles. Erin, Cyndi, Cara Lea, Sarah, Tansey, Lis, Yadeeh, Maria, Brit, 'Rie, Apple, Fred, Andee, & Shannon are my spiritual and girl-power strength. And finally to Travis, for your one shine. iii Acknowledgements I would like to thank my dissertation committee, Astrid Kodric-Brown, Tom Turner, Blair Wolf, & Joel Trexler. -

Plan De Manejo De La Reserva Natural Otamendi 2005-2009
Plan de Manejo de la Reserva Natural Otamendi 2005-2009 PLAN DE MANEJO DE LA RESERVA NATURAL OTAMENDI Administración de Parques Nacionales – Octubre 2004 Plan de Manejo de la Reserva Natural Otamendi 2005-2009 COORDINACIÓN GENERAL Gp. Jorge Juber Lic. Roberto Molinari Ing. Diana Uribelarrea COORDINACIÓN TÉCNICA Lic. Leonardo Raffo EQUIPO TÉCNICO DE PLANIFICACIÓN Prog. Asentamiento Humanos Mario Tomé, Graciela Antonietti Prog. Recursos Culturales Lorena Ferraro, Cecilia Uriarte, Paula Werber Prog. Recursos Naturales Fernanda Menvielle Liliana Goveto, Leonor Cusato Prog Uso Público Romina Casselli, Silvina Melhem, Raúl Romero, Reserva Natural Otamendi Walter Maciel, Marcelo Zanello, Leonardo Juber Prog de Planificación Rodolfo Burkart, Marcela Lunazzi Sistema de Información de Biodiversidad Martin Izquierdo, Federico Bava, Dirección de Educación e Interpretación Ambiental Pablo Reggio, Omar Tegaldo, Pilar García Conde, Andrea Valdosera Externos Oscar Trujillo COLABORACIONES Nora Nievas (ESCODELTA) Virginia De Francesco (AA/AOP) Santiago Krapovickas (AA/AOP) Andrés Bosso(AA/AOP) Eduardo Haene (AA/AOP) Javier Pereira (ACEN) Natalia Fracassi (ACEN) Diego Varela (CA) Marcos Ocampo Marcelo Canevari Karina De´Stefano Plan de Manejo de la Reserva Natural Otamendi 2005-2009 INDICE CAPITULO 1. Contexto del plan de manejo. Caracterización y valoración del área. Diagnóstico................................................................... 1 1 Contexto del manejo del área ................................................................ 1 1.1 Normativa