Protalix Biotherapeutics Appoints David Granot to Its Board of Directors
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Bigitech Fact Sheet 2017.12
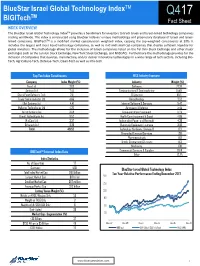
BlueStar Israel Global Technology IndexTM TM Q417 BIGITech Fact Sheet INDEX OVERVIEW The BlueStar Israel Global Technology IndexTM provides a benchmark for investors to track Israeli and Israel-linked technology companies trading worldwide. The index is constructed using BlueStar Indexes’ unique methodology and proprietary database of Israeli and Israel- linked companies. BIGITechTM is a modified market capitalization weighted index, capping the top-weighted constituents at 10%. It includes the largest and most liquid technology companies, as well as mid and small cap companies that display sufficient liquidity for global investors. The methodology allows for the inclusion of Israeli companies listed on the Tel Aviv Stock Exchange and other major exchanges such as the London Stock Exchange, New York Stock Exchange, and NASDAQ. Furthermore the methodology provides for the inclusion of companies that develop, manufacture, and/or deliver innovative technologies in a wide range of tech sectors, including Bio- Tech, Agriculture-Tech, Defense-Tech, Clean-Tech as well as Info-tech. Top Ten Index Constituents GICS Industry Exposure Company Index Weight (%) Industry Weight (%) Nice Ltd 7.63 Software 27.78 Amdocs Ltd 7.44 Semiconductors & Semiconductor 14.49 Check Point Software Tech 7.11 IT Services 10.15 Tower Semiconductor Ltd 4.66 Biotechnology 7.52 Elbit Systems Ltd 4.41 Internet Software & Services 5.47 Mellanox Technologies Ltd 4.26 Aerospace & Defense 5.45 Verint Systems Inc 3.89 Communications Equipment 5.24 Ormat Technologies Inc 3.52 Health Care Equipment & Suppli 4.86 Wix.Com Ltd 3.37 Independent Power and Renewabl 4.36 Orbotech Ltd 3.26 Electronic Equipment, Instrume 3.96 Total 49.52 Technology Hardware, Storage & 1.70 Diversified Financial Services 1.68 Pharmaceuticals 1.49 Hotels, Restaurants & Leisure 1.43 Machinery 1.38 BIGITech™ Internal Index Data Commercial Services & Supplies 0.69 Other 12.35 Index Statistics No. -

Ormat Technologies Inc
ORMAT TECHNOLOGIES INC MEETING DATE Wed, 06 Nov 2019 13:00 TYPE EGM ISSUE DATE Fri, 25 Oct 2019 MEETING LOCATION 6140 Plumas Street, Reno, NV 89519 CURRENT INDICES PIRC Global SECTOR Electric services COMPANY OVERVIEW Ormat Technologies is a provider of renewable power and energy technology. The Company is based in Reno, Nevada, and has over 150 power plants. MEETING SPECIFIC INFORMATION Ordinary resolutions are approved by simple majority of the votes cast. PROPOSALS ADVICE 1 Board Proposal to Declassify the Board For It is considered that staggered elections do not pursue shareholders’ best interest, as they entrench the board against hostile takeovers. In this sense, the Board’s proposal is welcomed as it will introduce annual election for all directors of the board, which is considered to be best practice. 2 Adjourn Meeting Oppose The Board requests authority to adjourn the special meeting until a later date or dates, if necessary, in order to permit further solicitation of proxies if there are not sufficient votes at the time of the special meeting to approve the merger. An oppose vote is recommended to any adjournment or postponement of meetings if a sufficient number of votes are present to constitute a quorum. It is considered that where a quorum is present, the vote outcome should be considered representative of shareholder opinion. ORMAT TECHNOLOGIES INC 06 Nov 2019 EGM 1 of 5 BOARD AND COMMITTEE COMPOSITION (post-Meeting) INDEPENDENT BY DIRECTOR GENDER PIRC COMPANY BOARD AC RC NC SC TENURE Dan Falk M No Yes NED C M M - 14 Todd C. -

Proxy Statement for Annual General Meeting of Shareholders 2019
GILAT SATELLITE NETWORKS LTD. Gilat House 21 Yegia Kapayim St. Kiryat Arye Petah Tikva 4913020, Israel ____________________ NOTICE OF ANNUAL GENERAL MEETING OF SHAREHOLDERS To be held on June 20, 2019 To our Shareholders: We cordially invite you to the Annual General Meeting of Shareholders of Gilat Satellite Networks Ltd. (the “Company”) to be held at the offices of the Company at 21 Yegia Kapayim Street, Kiryat Arye, Petah Tikva 4913020, Israel, on June 20, 2019 at 2 p.m. Israel time for the following purposes (the “Meeting”): 1. To set the number of directors serving on the Company Board at eight; 2. To re-elect six members of the Board of Directors until our next annual general meeting of shareholders and until their successors have been duly elected and qualified; 3. To approve an annual cash bonus plan for the Chief Executive Officer of the Company; 4. To approve a grant of options to the Chief Executive Officer of the Company; 5. To approve an annual cash bonus plan for the Chairman of the Company’s Board of Directors; 6. To amend our compensation policy for the Company’s directors and officers; and 7. To ratify and approve the reappointment and compensation of Kost Forer Gabbay & Kasierer, a member of Ernst & Young Global, as our independent registered public accountants for the fiscal year ending December 31, 2019, and for such additional period until the next annual general meeting of shareholders. In addition, our consolidated financial statements for the year ended December 31, 2018 will be received and considered at the Meeting. -

Bluestar Israel Equity Update First Quarter 2014
BlueStar Israel Equity Update First Quarter 2014 Q4 2013 in Review & 2014 Outlook All major global developed market equity benchmarks rose strongly in Q4 2013 despite the announcement of the Federal Reserve’s intentions to reduce the scope of its bond purchase What’s Inside program The BlueStar Israel Global Index rose 10.54% in Q4 2013 on an annualized basis while the TA-100 and MSCI Israel indices were up 7.38% and 6.34%, respectively. The BlueStar 1. Israeli Global benchmark also outperformed the TA-100 and MSCI Israel benchmarks for the full year 2013 by 4 and 15 percentage points, respectively. Equities in Q4 2013 For the second straight quarter the rally in Israeli equities was broad based and in each of the three largest sectors: Information Technology, Financials and Health Care. Additionally a long-awaited recovery in the telecom sector took hold during the quarter. Each of these sectors rose strongly during Q4 2013 indicating strength in the domestic economy and an 2.-6. About the BIGI & acceleration in the growth rates of the economies of Israel’s largest trading partners. BIGTech Indexes, The Israeli government budget deficit continued to shrink bringing on the prospect of lower Rebalance Review, taxes and higher government spending in 2014. The labor force participation rate rose in the and Risk/Return fourth quarter while the unemployment rate remained low. Data The revival in trading volume on the TASE, rise in equity prices in most developed countries, and several relatively large IPOs by Israeli companies during the second half of 2014 resulted in a net addition of 12 companies in the BlueStar Israel Global Index’s December rebalance. -

BIRD Newsletter Sep 2017
Dr. Eitan Yudilevich Executive Director More on 40 Years of Commitment to U.S. - Israel Collaboration In July 1977, at the U.S. Department of Commerce, the BIRD Foundation’s Board of Governors (BOG) held its inaugural meeting, marking the beginning of what would become a tradition of collaboration between U.S. and Israeli officials to discuss and select promising innovations, jointly presented by U.S. and Israeli companies. After 40 years, on June 13, 2017, the BOG met at a U.S. State Department facility, in Washington, D.C., followed by a great 40th Anniversary celebration at the National Museum of American History (more on the celebration in a dedicated article below). The day before the BOG meeting, representatives from the U.S. Department of Homeland Security (DHS) and the Ministry of Public Security (MOPS) met to discuss joint U.S.-Israeli projects submitted to the second annual cycle of the BIRD First Responders program. At the BOG meeting, 11 projects were approved, three of them for the DHS- MOPS First Responders program. In general, first responders are in great need of solutions to support safe, efficient and effective response to everyday emergencies. One important element of any modern solution includes the use of advanced technologies, properly developed or adapted to the varied and special circumstances in which first responders operate. The use of technology is now ubiquitous in the missions of individual infantry soldiers from advanced armies. However, the cost of equipment is still very high. For first responders, the need is not only to develop hi-tech solutions well matched to their missions, but also to take into consideration the affordability of such solutions. -

Ormat Sustainability Report 2018
ORMAT TECHNOLOGIES, INC. Putting the Earth First: SUSTAINABILITY REPORT 2018 Our Economic, Environmental and Social Performance Highlights Olkaria III geothermal complex, Kenya, 150 MW 1 CHAPTER I. A MESSAGE TO OUR STAKEHOLDERS Don A. Campbell geothermal complex, NV, U.S., 39 MW 2 3 This report demonstrates performance over time. Risk Management: sustainability progress. our Company’s sustainability Ormat places high value in compliance proposition, which is based on our Furthermore, this year’s report with all relevant regulations. Applying In summary, Ormat is pleased to dispersed portfolio of geothermal, represents our Company’s best practices in risk management submit this sustainability report solar, recovered energy and energy response to requests from our throughout our business is key to to you, our stakeholders. Our storage facilities that provide stakeholders to broaden our helping us achieve this goal. commitment to encouraging clean and renewable energy. Our disclosure on sustainability and the use of renewable power and renewable assets, which have little environmental initiatives and • Geothermal Resource (Brine and to reducing GHG emissions are emissions, mitigated 5.5 million adopt a global perspective on Steam) Management: embedded in Ormat’s corporate tons of GHG emissions in 2018, with sharing that information. Ormat employs sound, environmentally DNA. We are firmly committed to electricity generation totalling 5.9 prudent strategies to manage the these goals as a Company and as million Megawatt hours (MWh). As such, -

Israel Place
ENVIRONMENTAL SOCIAL E S G GOVERNANCE Israel Exposure Tilt For Professional Investor Use Only Copyright © 2019 Aperio Group, LLC Aperio v. [Latin] to make clear, to reveal the truth 103 Israel Exposure Tilt Overview Aperio Group has partnered with BlueStar Indexes® to develop an option that will tilt an Aperio portfolio toward companies with exposure to Israel. Companies are scored based on their level of involvement in Israel, reflecting a universe that includes: 1. Israeli companies,* 2. Non-Israeli companies with deep business ties and foreign direct investment, and 3. Non-Israeli companies with less significant business relationships. This hierarchy of scores is used to calculate a weighted-average score for a benchmark index and any potential portfolio. The portfolio is optimized to target the desired tilt toward this weighted-average score while replicating the risk characteristics of the benchmark as closely as possible. Our Israel Exposure Tilt can be used in combination with other Aperio strategies, exclusions, and/or tilts. *As defined by BlueStar. 2 For Professional Investor Use Only Israel Exposure Tilt BlueStar Pro-Israel Universe BlueStar assigns an Israel Exposure Score to the companies within the Aperio investable universe.+ Score Criteria 100 Israeli Companies* 75 Global Companies with a Major Presence in/Impact on Israel 50 Global Companies with a Material Presence in/Impact on Israel 0 Companies with No/Insignificant Involvement in Israel +Meets Aperio’s liquidity and capitalization requirements. *As defined by BlueStar. 3 For Professional Investor Use Only Israel Exposure Tilt BlueStar Methodology Score Term Qualification BlueStar takes an inclusionary view of what qualifies as an Israeli company, with a global perspective. -

Clean Energy Investing: Globla Comparison of Investment Returns
Clean Energy Investing: Global Comparison of Investment Returns March 2021 A Joint Report by the International Energy Agency and the Centre for Climate Finance & Investment Table of Contents 03 Executive Summary 05 Introduction 08 Analytical Methods 11 Key Investment Characteristics 14 Results 14 Global Markets 18 Advanced Economies 19 Emerging Market and Developing Economies 20 China 21 Transition Companies 22 The Covid Market Shock 24 Irrational Exuberance? 26 Conclusions 29 Acknowledgments 30 Annex A – Definition of Key Terms 32 Annex B – IEA Scenarios 33 Annex C – Fama-French Five-Factor Model 34 Annex D – Fossil Fuel Portfolio 48 Annex E – Renewable Power Portfolio 2 Executive Summary To shed light on the long-term prospects for clean energy, we investigate the historical financial performance of energy companies around the world in search of broad structural trends. This is the second in a series of joint reports by the International Energy Agency and Imperial College Business School examining the risk and return proposition in energy transitions. In this paper, we extend our coverage of publicly-traded renewable power and fossil fuel companies to the following: 1) global markets, 2) advanced economies, 3) emerging market and developing economies, and 4) China. We calculate the total return and annualized volatility of these portfolios over 5 and 10-year periods. Table 1 shows the 5 and 10-year results, up to December 31, 2020. Table 1 – Summary of Key Findings Global Markets Portfolios Advanced Economies Portfolios Fossil Fuel Renewable -

ORMAT TECHNOLOGIES, INC. (Exact Name of Registrant As Specified in Its Charter)
SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 10-Q ࠚ QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the quarterly period ended June 30, 2006 Or □ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the transition period from to Commission file number: 001-32347 ORMAT TECHNOLOGIES, INC. (Exact name of registrant as specified in its charter) DELAWARE 88-0326081 (State or other jurisdiction of (I.R.S. Employer incorporation or organization) Identification Number) 6225 Neil Road, Suite 300, Reno, Nevada 89511-1136 (Address of principal executive offices) Registrant’s telephone number, including area code: (775) 356-9029 Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ࠚ No □ Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, or a non-accelerated filer. See definition of ‘‘accelerated filer and large accelerated filer’’ in Rule 12b-2 of the Exchange Act. Large accelerated filer □ Accelerated filer ࠚ Non-accelerated filer □ Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes □ No ࠚ As of the date of this filing, the number of outstanding shares of common stock of Ormat Technologies, Inc. -

S&P Dow Jones Indices Announces Changes to the S&P / Harel Sector
S&P Dow Jones Indices announces changes to the S&P / Harel Sector Indices London, March 23, 2016 – The S&P / Harel Indices are being rebalanced after the close of trading on Thursday, March 31st. Below is the list of indices, their constituents and weights, effective on that date: S&P / Harel Consumer Goods Index Name Weight Osem Investment 15.00% Strauss Group 15.00% Delek Automotive Systems Ltd 15.00% Rami Levi Chain Stores Hashikma Marketing Ltd. 15.00% Delta-Galil Industries 15.00% Shufersal Ltd. 9.56% Neto ME Holdings Ltd 3.61% FOX WIZEL LTD 2.87% Kerur Hldgs 1 2.40% CARASSO MOTORS LTD 1.29% Maabarot Products Ltd. 1.27% Dor Alon Energy In Israel (1988) 1.00% Victory Supermarket Chain Ltd 1.00% Tiv Taam Holdings 1 Ltd. 1.00% Willy Food Investments Ltd. 1.00% S&P / Harel Energy Index Name Weight Isramco Negev 2 LP 15.00% Ormat Technologies 15.00% Paz Oil Company Ltd 15.00% Avner Oil & Gas Ltd LP 15.00% Delek Group Ltd 12.62% Delek Drilling LP 6.18% Oil Refineries Ltd 4.69% Ratio Oil Exploration L.P. 4.45% Jerusalem Oil Exploration 3.32% Delek Energy Systems Ltd 2.58% Energix - Renewable Energies Ltd 1.91% Naphtha Israel Petroleum Corp 1.24% Kenon Holdings Ltd. 1.00% I.N.O.C.-Dead Sea L.P. 1.00% Cohen Development & Industrial Buildings Ltd. 1.00% c S&P DOW JONES INDICES INDEX ANNOUNCEMENT S&P / Harel Health Care Index Name Weight Teva Pharmaceutical Industries 15.00% Perrigo Company plc 15.00% Mylan NV 15.00% Opko Health Inc 15.00% MannKind Corp 5.50% Compugen Ltd. -

Solactive-Bluestar Israel Economic Exposure Indexes Utilize the Constituents of Its Parent Index, the Bluestar Israel Global Index – Total Investable Market (BITM)
Solactive-BlueStar Israel Fact Sheet Economic Exposure Indexes Data as of Mar. 31, 2019 The Solactive-BlueStar Israel Domestic Exposure Index (IDEI) and Solactive-BlueStar Global Exposure Index (IGEI) provide investors with two distinct benchmarks for Israeli equities – designed to reflect the segmentation of the Israeli economy between globally-oriented firms and companies primarily focused on serving the domestic economy. These indexes can be used as vital indicators of Israel’s economic cycles and specific economic trends, as well as tools or vehicles to track the potential risk of geopolitical events in the volatile Middle East. Additionally, these indexes provide insight into the different risk, reward, correlation, yield and other technical and fundamental characteristics of Israeli Global Equities representing Israel’s domestic and global economies. The Solactive-BlueStar Israel Economic exposure indexes utilize the constituents of its parent index, the BlueStar Israel Global Index – Total Investable Market (BITM). The index methodologies define a company’s economic exposure by the percentage of its revenues derived from the target economic zone – revenue from within Israel for IDEI and revenue from outside of Israel for IGEI. In addition the index methodology provides for several diversification and liquidity limitations. Finally, the index methodology takes into account the importance of balanced, yet representative, sector exposure and diversification within sectors. 9-Year Performance 200 175 150 125 100 75 50 Mar-10 Mar-11 Mar-12 Mar-13 Mar-14 Mar-15 Mar-16 Mar-17 Mar-18 TA-125 BIGI MSCI EAFE Israel Domestic Exposure Israel Global Exposure www.bluestarindexes.com 1 Solactive-BlueStar Israel Domestic Exposure Fact Sheet Index Total Return (IDEITR) Data as of Mar. -

Ormat Sustainability Report 2019
ORMAT TECHNOLOGIES INC. SUSTAINABILITY REPORT 2019 Our Economic, Environmental and Social Performance Highlights Tungsten Mountain complex – 27 MW geothermal and 7AC solar - Nevada, U.S. 1 TABLE OF CONTENTS I. A MESSAGE FOR OUR STAKEHOLDERS ................................................................................................................................4 V. OUR HOLISTIC APPROACH TO CORPORATE GOVERNANCE ...........................................................................................52 II. HOW ORMAT POWERS OUR RENEWABLE ENERGY FUTURE ..........................................................................................8 Making Sound Corporate Governance a Priority ................................................................................................................................54 Ormat: What We Do ...................................................................................................................................................................................14 Ormat’s Corporate Governance Structure.................................................................................................................................................54 About Our Renewable Energy Business .....................................................................................................................................................14 Our Corporate Governance Policies and Guidelines .............................................................................................................................59