Effects of Post-Glacial Range Expansions and Population Bottlenecks on Species Richness
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
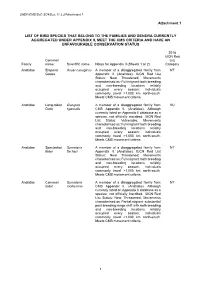
Attachment 1 LIST of BIRD SPECIES THAT BELONG to the FAMILIES
UNEP/CMS/ScC-SC4/Doc.11.3.2/Attachment 1 Attachment 1 LIST OF BIRD SPECIES THAT BELONG TO THE FAMILIES AND GENERA CURRENTLY AGGREGATED UNDER APPENDIX II, MEET THE CMS CRITERIA AND HAVE AN UNFAVOURABLE CONSERVATION STATUS 2018 IUCN Red Common List Family name Scientific name Notes for Appendix II (Sheets 1 or 2) Category Anatidae Emperor Anser canagicus A member of a disaggregated family from NT Goose Appendix II. (Anatidae). IUCN Red List Status: Near Threatened; Movements characterised as: Full migrant: both breeding and non-breeding locations reliably occupied every season; individuals commonly travel >1,000 km north-south. Meets CMS movement criteria. Anatidae Long-tailed Clangula A member of a disaggregated family from VU Duck hyemalis CMS Appendix II. (Anatidae). Although currently listed on Appendix II database as a species, not officially inscribed. IUCN Red List Status: Vulnerable; Movements characterised as: Full migrant: both breeding and non-breeding locations reliably occupied every season; individuals commonly travel >1,000 km north-south. Meets CMS movement criteria. Anatidae Spectacled Somateria A member of a disaggregated family from NT Eider fischeri Appendix II. (Anatidae). IUCN Red List Status: Near Threatened; Movements characterised as: Full migrant: both breeding and non-breeding locations reliably occupied every season; individuals commonly travel >1,000 km north-south. Meets CMS movement criteria. Anatidae Common Somateria A member of a disaggregated family from NT Eider mollissima CMS Appendix II. (Anatidae). Although currently listed on Appendix II database as a species, not officially inscribed. IUCN Red List Status: Near Threatened; Movements characterised as: Partial migrant: substantial post-breeding range shift with both breeding and non-breeding locations reliably occupied every season; individuals commonly travel >1,000 km north-south. -

Predicting the Distribution of the Vulnerable Yellow-Breasted Pipit (Anthus Chloris) Using Species Distribution Modelling
1 Predicting the distribution of the Vulnerable Yellow-breasted Pipit (Anthus chloris) using Species Distribution Modelling Darren W. Pietersen1*, Ian T. Little2, Raymond Jansen3 and Andrew E. McKechnie1 1DST-NRF Centre of Excellence at the Percy FitzPatrick Institute, Department of Zoology and Entomology, University of Pretoria, Private Bag X20, Hatfield, 0028, South Africa 2The Endangered Wildlife Trust, Private Bag X11, Modderfontein, 1645, Johannesburg, South Africa 3Department of Environmental, Water and Earth Sciences, Tshwane University of Technology, Private Bag X680, Pretoria, 0001, South Africa *Address for correspondence: Department of Zoology and Entomology University of Pretoria Private Bag X20 Hatfield 0028 [email protected] +27 82 937 6052 2 Abstract The Yellow-breasted Pipit (Anthus chloris) is endemic to the eastern escarpment of South Africa, marginally entering eastern Lesotho. This species is classified as globally Vulnerable due to a perceived decreasing population size and loss of habitat. We employed Species Distribution Modelling to investigate the predicted range of this species to determine whether additional purportedly suitable habitat exists where this species may be found, and to assess the degree to which habitat loss has affected this species. We used a database of 250 independently obtained and verified sightings to predict the summer breeding distribution of this species and compare our verified sightings and predicted range to the sightings currently in the Second Southern African Bird Atlas Project (SABAP2) database and the latest regional conservation assessment. Our models closely approximate the current distribution of the Yellow-breasted Pipit, and suggest that most of the purportedly suitable habitat is occupied, at least at the macro-scale. -

Bontebok Birds
Birds recorded in the Bontebok National Park 8 Little Grebe 446 European Roller 55 White-breasted Cormorant 451 African Hoopoe 58 Reed Cormorant 465 Acacia Pied Barbet 60 African Darter 469 Red-fronted Tinkerbird * 62 Grey Heron 474 Greater Honeyguide 63 Black-headed Heron 476 Lesser Honeyguide 65 Purple Heron 480 Ground Woodpecker 66 Great Egret 486 Cardinal Woodpecker 68 Yellow-billed Egret 488 Olive Woodpecker 71 Cattle Egret 494 Rufous-naped Lark * 81 Hamerkop 495 Cape Clapper Lark 83 White Stork n/a Agulhas Longbilled Lark 84 Black Stork 502 Karoo Lark 91 African Sacred Ibis 504 Red Lark * 94 Hadeda Ibis 506 Spike-heeled Lark 95 African Spoonbill 507 Red-capped Lark 102 Egyptian Goose 512 Thick-billed Lark 103 South African Shelduck 518 Barn Swallow 104 Yellow-billed Duck 520 White-throated Swallow 105 African Black Duck 523 Pearl-breasted Swallow 106 Cape Teal 526 Greater Striped Swallow 108 Red-billed Teal 529 Rock Martin 112 Cape Shoveler 530 Common House-Martin 113 Southern Pochard 533 Brown-throated Martin 116 Spur-winged Goose 534 Banded Martin 118 Secretarybird 536 Black Sawwing 122 Cape Vulture 541 Fork-tailed Drongo 126 Black (Yellow-billed) Kite 547 Cape Crow 127 Black-shouldered Kite 548 Pied Crow 131 Verreauxs' Eagle 550 White-necked Raven 136 Booted Eagle 551 Grey Tit 140 Martial Eagle 557 Cape Penduline-Tit 148 African Fish-Eagle 566 Cape Bulbul 149 Steppe Buzzard 572 Sombre Greenbul 152 Jackal Buzzard 577 Olive Thrush 155 Rufous-chested Sparrowhawk 582 Sentinel Rock-Thrush 158 Black Sparrowhawk 587 Capped Wheatear -

Interspecific Social Dominance Mimicry in Birds
bs_bs_banner Zoological Journal of the Linnean Society, 2014. With 6 figures Interspecific social dominance mimicry in birds RICHARD OWEN PRUM1,2* 1Department of Ecology and Evolutionary Biology, Yale University, New Haven, CT 06520-8150, USA 2Peabody Natural History Museum, Yale University, New Haven, CT 06520-8150, USA Received 3 May 2014; revised 17 June 2014; accepted for publication 21 July 2014 Interspecific social dominance mimicry (ISDM) is a proposed form of social parasitism in which a subordinate species evolves to mimic and deceive a dominant ecological competitor in order to avoid attack by the dominant, model species. The evolutionary plausibility of ISDM has been established previously by the Hairy-Downy game (Prum & Samuelson). Psychophysical models of avian visual acuity support the plausibility of visual ISDM at distances ∼>2–3 m for non-raptorial birds, and ∼>20 m for raptors. Fifty phylogenetically independent examples of avian ISDM involving 60 model and 93 mimic species, subspecies, and morphs from 30 families are proposed and reviewed. Patterns of size differences, phylogeny, and coevolutionary radiation generally support the predic- tions of ISDM. Mimics average 56–58% of the body mass of the proposed model species. Mimics may achieve a large potential deceptive social advantage with <20% reduction in linear body size, which is well within the range of plausible, visual size confusion. Several, multispecies mimicry complexes are proposed (e.g. kiskadee- type flycatchers) which may coevolve through hierarchical variation in the deceptive benefits, similar to Müllerian mimicry. ISDM in birds should be tested further with phylogenetic, ecological, and experimental investigations of convergent similarity in appearance, ecological competition, and aggressive social interactions between sympatric species. -

South Africa Mega Birding III 5Th to 27Th October 2019 (23 Days) Trip Report
South Africa Mega Birding III 5th to 27th October 2019 (23 days) Trip Report The near-endemic Gorgeous Bushshrike by Daniel Keith Danckwerts Tour leader: Daniel Keith Danckwerts Trip Report – RBT South Africa – Mega Birding III 2019 2 Tour Summary South Africa supports the highest number of endemic species of any African country and is therefore of obvious appeal to birders. This South Africa mega tour covered virtually the entire country in little over a month – amounting to an estimated 10 000km – and targeted every single endemic and near-endemic species! We were successful in finding virtually all of the targets and some of our highlights included a pair of mythical Hottentot Buttonquails, the critically endangered Rudd’s Lark, both Cape, and Drakensburg Rockjumpers, Orange-breasted Sunbird, Pink-throated Twinspot, Southern Tchagra, the scarce Knysna Woodpecker, both Northern and Southern Black Korhaans, and Bush Blackcap. We additionally enjoyed better-than-ever sightings of the tricky Barratt’s Warbler, aptly named Gorgeous Bushshrike, Crested Guineafowl, and Eastern Nicator to just name a few. Any trip to South Africa would be incomplete without mammals and our tally of 60 species included such difficult animals as the Aardvark, Aardwolf, Southern African Hedgehog, Bat-eared Fox, Smith’s Red Rock Hare and both Sable and Roan Antelopes. This really was a trip like no other! ____________________________________________________________________________________ Tour in Detail Our first full day of the tour began with a short walk through the gardens of our quaint guesthouse in Johannesburg. Here we enjoyed sightings of the delightful Red-headed Finch, small numbers of Southern Red Bishops including several males that were busy moulting into their summer breeding plumage, the near-endemic Karoo Thrush, Cape White-eye, Grey-headed Gull, Hadada Ibis, Southern Masked Weaver, Speckled Mousebird, African Palm Swift and the Laughing, Ring-necked and Red-eyed Doves. -

Multi-Locus Phylogeny of African Pipits and Longclaws (Aves: Motacillidae) Highlights Taxonomic Inconsistencies
Running head: African pipit and longclaw taxonomy Multi-locus phylogeny of African pipits and longclaws (Aves: Motacillidae) highlights taxonomic inconsistencies DARREN W. PIETERSEN,1* ANDREW E. MCKECHNIE,1,2 RAYMOND JANSEN,3 IAN T. LITTLE4 AND ARMANDA D.S. BASTOS5 1DST-NRF Centre of Excellence at the Percy FitzPatrick Institute, Department of Zoology and Entomology, University of Pretoria, Hatfield, South Africa 2South African Research Chair in Conservation Physiology, National Zoological Garden, South African National Biodiversity Institute, P.O. Box 754, Pretoria 0001, South Africa 3Department of Environmental, Water and Earth Sciences, Tshwane University of Technology, Pretoria, South Africa 4Endangered Wildlife Trust, Johannesburg, South Africa 5Department of Zoology and Entomology, University of Pretoria, Hatfield, South Africa *Corresponding author. Email: [email protected] 1 Abstract The globally distributed avian family Motacillidae consists of 5–7 genera (Anthus, Dendronanthus, Tmetothylacus, Macronyx and Motacilla, and depending on the taxonomy followed, Amaurocichla and Madanga) and 66–68 recognised species, of which 32 species in four genera occur in sub- Saharan Africa. The taxonomy of the Motacillidae has been contentious, with variable numbers of genera, species and subspecies proposed and some studies suggesting greater taxonomic diversity than what is currently (five genera and 67 species) recognised. Using one nuclear (Mb) and two mitochondrial (cyt b and CO1) gene regions amplified from DNA extracted from contemporary and museum specimens, we investigated the taxonomic status of 56 of the currently recognised motacillid species and present the most taxonomically complete and expanded phylogeny of this family to date. Our results suggest that the family comprises six clades broadly reflecting continental distributions: sub-Saharan Africa (two clades), the New World (one clade), Palaearctic (one clade), a widespread large-bodied Anthus clade, and a sixth widespread genus, Motacilla. -

SOUTH AFRICA: LAND of the ZULU 26Th October – 5Th November 2015
Tropical Birding Trip Report South Africa: October/November 2015 A Tropical Birding CUSTOM tour SOUTH AFRICA: LAND OF THE ZULU th th 26 October – 5 November 2015 Drakensberg Siskin is a small, attractive, saffron-dusted endemic that is quite common on our day trip up the Sani Pass Tour Leader: Lisle Gwynn All photos in this report were taken by Lisle Gwynn. Species pictured are highlighted RED. 1 www.tropicalbirding.com +1-409-515-0514 [email protected] Page Tropical Birding Trip Report South Africa: October/November 2015 INTRODUCTION The beauty of Tropical Birding custom tours is that people with limited time but who still want to experience somewhere as mind-blowing and birdy as South Africa can explore the parts of the country that interest them most, in a short time frame. South Africa is, without doubt, one of the most diverse countries on the planet. Nowhere else can you go from seeing Wandering Albatross and penguins to seeing Leopards and Elephants in a matter of hours, and with countless world-class national parks and reserves the options were endless when it came to planning an itinerary. Winding its way through the lush, leafy, dry, dusty, wet and swampy oxymoronic province of KwaZulu-Natal (herein known as KZN), this short tour followed much the same route as the extension of our South Africa set departure tour, albeit in reverse, with an additional focus on seeing birds at the very edge of their range in semi-Karoo and dry semi-Kalahari habitats to add maximum diversity. KwaZulu-Natal is an oft-underrated birding route within South Africa, featuring a wide range of habitats and an astonishing diversity of birds. -

Scientific Results of the Vernay Lang Kalahari Expedition Birds.Pdf
ANNALE ANNALS VAN DIE OF THE TRANSVAAL MUSEUM VOL. 16 PART I SCIENTIFIC RESULTS OF THE VERNAY- LANG KALAHARI EXPEDITION, MARCH TO SEPTEMBER, 1930 BIRDS By AUSTIN ROBERTS With Plate I HE Vernay-Lang Kalahari Expedition had its inception towards Tthe end of the year 1930, when .:vIr A. S. Vernay arranged with .:vIr Herbert Lang (who was then a guest of the Transvaal .:vIuseum) to combine a sporting and scientific expedition across the unknown central Kalahari to Ngamiland. Mr Vernay had on previous occasions under- taken expeditions for the purpose of securing desiderata in big game animals for museums, and on one occasion Mr Lang had accompanied him and secured much other zoological material for study purposes in Angola. Mr Lang was specially qualified to undertake explorations of this nature from earlier experiences when engaged by the American Museum of Natural History, in which he was Associate Curator of Mammalogy, in collecting specimens in East Africa and the north-eastern Congo. His extraordinary success in the accomplishment of these enterprises had already earned for him a world-wide reputation, and to these successes may be added the present one, as the detailed reports will show when published in due course. Mr Lang has been able to demonstrate that it is not the acquisition of only a few specimens, which become the jealously guarded possessions of a single institution, that makes the suc- cess of an expedition, but tbat by the employment of men who know their business and by their careful collecting of material in such quantity as the particular cases warrant, important facts of value to science can be accumulated. -

Diversity, Distribution and Habitat Association of Birds in Menze-Guassa Community Conservation Area, Central Ethiopia
Vol. 10(9), pp. 372-379, September 2018 DOI: 10.5897/IJBC2018.1196 Article Number: 883998C58352 ISSN: 2141-243X Copyright ©2018 International Journal of Biodiversity and Author(s) retain the copyright of this article http://www.academicjournals.org/IJBC Conservation Full Length Research Paper Diversity, distribution and habitat association of birds in Menze-Guassa Community Conservation Area, Central Ethiopia Yihenew Aynalem* and Bezawork Afework Department of Zoological Sciences, College of Natural and Computational Sciences, Addis Ababa University, Addis Ababa, Ethiopia. Received 27 April, 2018; Accepted 14 June, 2018 A study was conducted in Menz-Guassa Community Conservation Area (MGCCA) from November 2016 to March 2017, to assess the diversity, distribution and habitat association of birds. Three habitat types including forest, grassland, and moorland habitats were identified based on their vegetation composition. Point count method in Eucalyptus and Juniperus forest, and line transect technique in grassland and moorland habitats were used to study avian diversity. Data were collected in the early morning (6:30 to 9:30 a.m.) and late afternoon (4:30 to 7:00 p.m.) when the activities of birds were prominent. Species diversity and evenness was given in terms of Shannon-Weaver diversity Index. A total of 86 avian species belonging to 14 orders and 35 families were identified. The identified areas are rich with seven (8.14%) endemic bird species namely; abyssinian catbird (Parophasma galinieri), abyssinian longclaw (Macronyx flavicollis), ankober serin (Crithagra ankoberensis), black-headed siskin (Serinus nigriceps), blue-winged goose (Cyanochen cyanoptera), moorland francolin (Scleroptlia psilolaema), spot-breasted plover (Vanellus melanocephalus), and five (5.81%) near-endemic bird species including rouget's rail (Rougetius rougetii), wattled ibis (Bostrychia carunculata), white-collared pigeon (Columba albitorques), thick-billed raven (Corvus crassirostris), and white-winged cliff chat (Myrmecocichla semirufa). -

Ultimate Zambia (Including Pitta) Tour
BIRDING AFRICA THE AFRICA SPECIALISTS Ultimate Zambia including Zambia Pitta 2019 Tour Report © Yann Muzika © Yann African Pitta Text by tour leader Michael Mills Photos by tour participants Yann Muzika, John Clark and Roger Holmberg SUMMARY Our first Ultimate Zambia Tour was a resounding success. It was divided into three more manageable sections, namely the North-East Extension, © John Clark © John Main Zambia Tour and Zambia Pitta Tour, each with its own delights. Birding Africa Tour Report Tour Africa Birding On the North-East Pre-Tour we started off driving We commenced the Main Zambia Tour at the Report Tour Africa Birding north from Lusaka to the Bangweulu area, where spectacular Mutinondo Wilderness. It was apparent we found good numbers of Katanga Masked that the woodland and mushitu/gallery forest birds Weaver coming into breeding plumage. Further were finishing breeding, making it hard work to Rosy-throated Longclaw north at Lake Mweru we enjoyed excellent views of track down all the key targets, but we enjoyed good Zambian Yellow Warbler (split from Papyrus Yellow views of Bar-winged Weaver and Laura's Woodland Warbler) and more Katanga Masked Weavers not Warbler and found a pair of Bohm's Flycatchers finally connected with a pair of Whyte's Francolin, Miombo Tit, Bennett's Woodpecker, Amur Falcon, yet in breeding plumage. From here we headed east feeding young. Other highlights included African which we managed to flush. From Mutinondo Cuckoo Finch, African Scops Owl, White-crested to the Mbala area we then visited the Saisi River Barred Owlet, Miombo Rock Thrush and Spotted we headed west with our ultimate destination as Helmetshrike and African Spotted Creeper. -

16-DAY SUBTROPICAL SOUTH AFRICA TRIP REPORT, 10 – 25 March 2017
SOUTH AFRICA: 16‐DAY SUBTROPICAL SOUTH AFRICA TRIP REPORT, 10 – 25 March 2017 By Jason Boyce Drakensberg Rockjumper – One of the birds of the trip! www.birdingecotours.com [email protected] 2 | T R I P R E P O R T Subtropical South Africa Trip Report March 2017 TOUR ITINERARY Overnight Day 1 – Arrival and birding Umhlanga Gateway Country Lodge, Umhlanga Day 2 – Umhlanga to Underberg KarMichael Guest Farm, Himeville Day 3 – Sani Pass KarMichael Guest Farm, Himeville Day 4 – Southern Drakensberg to Eshowe Birds of Paradise B&B, Eshowe Day 5 – Ongoye, Mtunzini and Amatikulu Birds of Paradise B&B, Eshowe Day 6 – Eshowe, Dlinza to St Lucia Ndiza Lodge, St Lucia Day 7 – St Lucia Wetland Park Ndiza Lodge, St Lucia Day 8 – St Lucia to Mkhuze Game Reserve Mantuma Camp, Mkhuze Day 9 – Mkhuze Game Reserve Mantuma Camp, Mkhuze Day 10 – Mkhuze to Wakkerstroom Wetlands Country House, Wakkerstroom Day 11 – Wakkerstroom birding Wetlands Country House, Wakkerstroom Day 12 – Wakkerstroom to Skukuza, KNP Kruger National Park, Skukuza Day 13 – Southern Kruger National Park Kruger National Park, Skukuza Day 14 – Kruger National Park to Dullstroom Linger Longer, Dullstroom Day 15 – Dullstroom to Dinokeng Game Reserve Leopardsong Game Lodge, Dinokeng Day 16 – Rust de Winter to Johannesburg airport Flight home OVERVIEW This was a tour with incredible diversity, varying habitats, enjoyable company, and a host of endemic South African bird species. Our 16-day ‘Subtropical South Africa’ tour gave us 397 species of birds, with an additional 15 species being heard only. We also saw 37 mammal species, interesting reptiles, and a few rare South African butterflies. -

Kenya November 2019
Tropical Birding Trip Report KENYA NOVEMBER 2019 Kenya: The Coolest Trip in Africa 10th – 26th November, 2019 TOUR LEADER: Charley Hesse. Report & photos by Charley Hesse. All photos were taken on this tour. Kenya offers a wide variety of landscapes and habitats with a corresponding long list of bird and mammal species. We started this tour in Kenya’s capital Nairobi, and the Nairobi National Park where we saw dancing Grey Crowned Cranes and an amazing 9 species of cisticola in a day, with the bizarre backdrop of the city’s skyline. We dipped down the Magadi road into very dry scrub with a totally different set of species like Fischer’s Sparrow-Larks & Cut- throats. From Nairobi we drove to the foothills of Mt Kenya and in the lush montane forest saw the huge Crowned Eagle and verdant Hartlaub’s Turaco. Next, we visited the Aberdares NP and explored the alpine moorland where we saw the huge Jackson’s Francolin and endemic Aberdare Cisticola. At the bizarre Ark Lodge, we saw Giant Forest Hog, plus genets and galago at night. We dropped down from the highlands into the great rift valley and explored Lake Nakuru and Lake Naivasha with their myriad of water birds. The boat ride at Lake Naivasha was particularly good for photography. On to Lake Baringo with its amazing local guides who have many great birds staked out including owls, nightjars and coursers. Another boat ride produced White-backed Night-Heron and Northern Carmine Bee-eater. At Kakamega we explored the lush forest and found specialties like turacos, robin- chats, batises and the African Broadbill.