The Smart-Phone Application Follow-Up System for Medication Compliance in Patients with Depression
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mitsubishi Electric and Zhuzhou CSR Times Electronic Win Order for Beijing Subway Railcar Equipment
FOR IMMEDIATE RELEASE No. 2496 Product Inquiries: Media Contact: Overseas Marketing Division, Public Utility Systems Group Public Relations Division Mitsubishi Electric Corporation Mitsubishi Electric Corporation Tel: +81-3-3218-1415 Tel: +81-3-3218-3380 [email protected] [email protected] http://global.mitsubishielectric.com/transportation/ http://global.mitsubishielectric.com/news/ Mitsubishi Electric and Zhuzhou CSR Times Electronic Win Order for Beijing Subway Railcar Equipment Tokyo, January 13, 2010 – Mitsubishi Electric Corporation (TOKYO: 6503) announced today that Mitsubishi Electric and Zhuzhou CSR Times Electronic Co., Ltd. have received orders from Beijing MTR Construction Administration Corporation for electric railcar equipment to be used on the Beijing Subway Changping Line. The order, worth approximately 3.6 billion yen, comprises variable voltage variable frequency (VVVF) inverters, traction motors, auxiliary power supplies, regenerative braking systems and other electric equipment for 27 six-coach trains. Deliveries will begin this May. The Changping Line is one of five new subway lines scheduled to start operating in Beijing this year. The 32.7-kilometer line running through the Changping district of northwest Beijing will have 9 stops between Xierqi and Ming Tombs Scenic Area stations. Mitsubishi Electric’s Itami Works will manufacture traction motors for the 162 coaches. Zhuzhou CSR Times Electronic will make the box frames and procure certain components. Zhuzhou Shiling Transportation Equipment Co., Ltd, a joint-venture between the two companies, will assemble all components and execute final testing. Mitsubishi Electric already has received a large number of orders for electric railcar equipment around the world. In China alone, orders received from city metros include products for the Beijing Subway lines 2 and 8; Tianjin Metro lines 1, 2 and 3; Guangzhou Metro lines 4 and 5; and Shenyang Metro Line 1. -

2019-07Contents
Full Text and Manuscript Submission: www.besjournal.com BES Biomedical and Environmental Sciences Table of Contents July 2019, Volume 32, Number 7 Development of Public Health for 70 Years 477 Association between Lipoprotein (a) Levels and Metabolic Syndrome in a Middle-aged and Elderly Chinese Cohort WU Xue Yan, LIN Lin, Qi Hong Yan, DU Rui, HU Chun Yan, MA Li Na, PENG Kui, LI Mian, XU Yu, XU Min, CHEN Yu Hong, LU Jie Li, BI Yu Fang, WANG Wei Qing, and NING Guang 485 Shanghai Institute of Endocrinology and Metabolism Original Articles 486 Food Frequency Questionnaire for Chinese Children Aged 12-17 Years: Validity and Reliability LIU Dan, JU La Hong, YANG Zhen Yu, ZHANG Qian, GAO Jian Fen, GONG Di Ping, GUO Dan Dan, LUO Shu Quan, and ZHAO Wen Hua 496 The Impacts of Simulated Microgravity on Rat Brain Depended on Durations and Regions CHEN Bo, ZHANG Yu Shi, LI George, CHO Jun-Lae, DENG Yu Lin, and LI Yu Juan 508 Subchronic Oral Cadmium Exposure Exerts both Stimulatory and Suppressive Effects on Pulmonary Inflammation/Immune Reactivity in Rats KULAS Jelena, NINKOV Marina, TUCOVIC Dina, POPOV Aleksandrov Aleksandra, UKROPINA Mirela, CAKIC MILOSEVIC Maja, MUTIC Jelena, KATARANOVSKI Milena, and MIKROV Ivana 520 An Investigation on the Molecular Characteristics and Intracellular Growth Ability among Environmental and Clinical Isolates of Legionella pneumophila in Sichuan Province, China ZENG Lin Zi, LIAO Hong Yu, LUO Long Ze, HE Shu Sen, QIN Tian, ZHOU Hai Jian, LI Hong Xia, CHEN Da Li, and CHEN Jian Ping 531 Intranasal Immunization Using -

Beijing's Suburbs
BEIJING MUNICIPAL COmmISSION OF TOURISM DEVELOPMENT BEIJING’S SUBURBS & SMALL TOWNS TO VISIT Getaway from China’s Capital —— 1 Discovering the Unique Charm and Vibes of Beijing’s Suburbs and Small Towns 1 Beijing’s Suburban Charm and Small-Town Vibes In the long-standing imperial Beijing, the red walls and yellow tiles exude the majestic imperial glamour, and the sedate country scene easily comes into your peripheral vision. A visit in Beijing guarantees you a walk of imperial solemnity in downtown Beijing, and a lot more country fun in the suburbs. You will see the many faces of the suburbs in the four seasons, walk through all the peaceful folk villages and exotic small towns, and make the most of your Beijing trips. This feature will highlight attractions of Beijing’s suburbs in the four seasons and open up year-round opportunities for visitors to soak up the best of the country life. A variety of small towns will also be featured, making for the best short trips to relax. 2 TRAVEL IN BEIJING’S SUBURBS AND SMALL TOWNS Highlights A Travel Guide to Beijing’s Suburbs Spring Explore the Nature | Feast on the Wild Summer Make a Splash | Go on Leisurely Outings Autumn Hike for Foliage | Foraging for Autumn Fruits Winter Ski down the Slopes | Bathe in Hot Springs 3 Best Small Towns to Visit “Chinese national” Small Towns 2 Gubei Water Town the Ultimate Retreat | Xiaotangshan the Hot Spring Resort “Western style” Small Towns 2 Spring Legend Town in Huairou | Huanghou Town Leisure Holiday Village Themed Small Towns 3 CTSHK RV Park of MYNS | Chateau Changyu AFIP Global Beijing | Qianjiadian Town in Yanqing Unique Cultural Villages 3 Cuandixia Village | Lingshui Village in Mentougou | Kangling Village For more information, please see the details below. -

P020160825659207952288.Pdf
Table of Content I. Introduction to the Project....................................................................................................................................... 1 II. Introduction to CUPL..............................................................................................................................................4 III. Introduction to the Second Training Session...................................................................................................... 5 III.A. Venue............................................................................................................................................................5 III.B. Curriculum.................................................................................................................................................. 5 III.C. Introduction to Speakers and Title of the Courses.................................................................................6 III.D. Name List of the Participants of the Second Training Session............................................................ 7 III.E. Opening Ceremony.................................................................................................................................... 8 III.F. Closing Ceremony....................................................................................................................................... 9 III.G. Note on Attendance.................................................................................................................................. -

1 AMAN SUMMER PALACE AMANYARA Meetings & Corporate Events 1 AMANYARA Meetings & Corporate Events 1
AMANAMANYARA SUMMER Meetings PALACE & Corporate Events 1 A land that has mystified and fascinated outsiders Activities Guide 中国作为魅力十足的神秘东方古国,数百年来吸引 for centuries, China boasts one of the world’s oldest 着世界游客心驰神往。中国拥有世界上最古老的 civilisations and some of its most recognisable 文明之一和最著名的人工奇迹,如远隔数千年的公 活动指南 landmarks, including the Great Wall. This iconic 元前世纪便屹立在首都北京北部山区的标志性建 structure straddles the mountains north of Beijing, 筑——长城。经过历史朝代的更迭重建,中国拥有 China’s capital, an ancient city dating from the first 众多被列入联合国教科文组织世界遗产名录的名 millennium BC. Rebuilt multiple times through its 胜古迹。如今,北京正以日新月异的速度持续焕发 dynastic history and home to numerous Unesco World 生机。 Heritage Sites, today Beijing’s renewal continues apace. 璀璨的古代文明与时尚的现代气息在北京得以完 A visit to Beijing offers travellers a kaleidoscope of 美的融合。自明朝(1368-1644)开始,北京的城市 old and new. With the Forbidden City at its heart, the 布局便以紫禁城为中心向四周以环形扩散展开。古 capital expands in concentric rings echoing a blueprint 代北京在庙宇和宫殿间留出的狭窄小路便是人们 from the Ming dynasty (1368-1644). Old Beijing is 熟知的胡同,如今北京二环路内偶然可见的残垣 found in the temples, palaces and narrow alleyways 断壁便是早已拆除的城墙原址。 AMAN SUMMER PALACE 2 known as hutongs within the city’s second ring road, 这座历史悠久的城市中心的附近区域却生动地展 the original site of the long-demolished city walls. 现了当代中国的繁荣景象。高耸入云的摩天大楼; 从美术馆,歌剧院到美食餐厅,绚烂多彩的文化和 Around this historic centre, the modern city is a 娱乐选择使这座城市丰富多彩。以闻名遐迩的颐 living showcase of contemporary China. Skyscrapers 和园命名,颐和安缦提供私密优享的入园通道进 reach for the heavens and the city’s rich culture is 入皇家园林。颐和安缦地理位置优越,方便客人前 celebrated with a wealth of cultural and entertainment 往宁静的古长城,距离故宫和北京市中心仅20公里 options, from art galleries and opera houses, to fine ( 约 4 5 分 钟 车 程 )。 dining. Situated alongside the Unesco-protected site that shares its name, Aman Summer Palace provides unparalleled access to this historic garden estate through a private entrance. -

2013 DI ASIA PACIFIC BEIJING INVITATIONAL Welcome to Beijing
2013 DI ASIA PACIFIC BEIJING INVITATIONAL December 5th-9th, 2013 Beijing, China Welcome to Beijing China TABLE OF CONTENTS THE INVITATION Page 3 GETTING STARTED Page 4 AT THE TOURNAMENT Page 6 GETTING IN AND OUT OF BEIJING Page 7 TRAVELING IN BEIJING Page 8 APPLICATION FORM Page 10 2 THE INVITATION September 29, 2013 Dear DI International teams, It is with pride and pleasure that I invite you to 2013 DI Asia Pacific Beijing Invitational, to be held on December 5th-9th, 2013. Over 400 teams qualified from China Regional Tournaments will attend this year’s event. Your participation means that you will take part in the biggest event of youth creativity in Beijing, China with thousands of Chinese talents. The event will be held at “Hot Spring Leisure City”, one of the top spa resorts in Beijing. The facilities will provide all teams from around the globe with the best services possible. All registered international teams will automatically enter the special National Geographic Challenge, while the Team Challenges are optional. Teams will also have many opportunities to take part in a variety of special events during the duration of the Tournament. We expect that the 2013 DI Asia Pacific Beijing invitational to be the largest youth event in China. We also hope the Tournament will offer you remarkable and unforgettable cultural experiences that will give you the opportunity to explore Beijing. The following information will provide you the details of the event. We strongly suggest that you read carefully through this information. If you have further questions, please do not hesitate to contact DI China at [email protected]. -
![Directors and Parties Involved in the [Redacted]](https://docslib.b-cdn.net/cover/0981/directors-and-parties-involved-in-the-redacted-2180981.webp)
Directors and Parties Involved in the [Redacted]
THIS DOCUMENT IS IN DRAFT FORM, INCOMPLETE AND SUBJECT TO CHANGE AND THAT THE INFORMATION MUST BE READ IN CONJUNCTION WITH THE SECTION HEADED “WARNING” ON THE COVER OF THIS DOCUMENT DIRECTORS AND PARTIES INVOLVED IN THE [REDACTED] DIRECTORS Name Residential Address Nationality Executive Directors Mr. Shi Wei (施煒) 2035, District C, Yosemite Chinese Wenyuzhuangyuan Shunyi District Beijing PRC Mr. Yang Weimin (楊為民) 2-005, Meiguiyuan Road Chinese Baolilongshang No. 9, Litang Road Tangshan Town Changping District, Beijing PRC Mr. Wang Liang (王亮) Building 307, Yujing Garden Chinese Yuyang Road, Houshayu Shunyi District, Beijing PRC Mr. He Jiyong (賀繼永) No. 14 Zhongnan Road Chinese Wuchang District, Wuhan Hubei Province PRC Mr. Wang Wei (王偉) 2401 Building 2 Chinese Yijiajiayuan, No. 8 Yard Tonghui South Road Tongzhou District Beijing PRC Mr. Sui Huijun (眭輝俊) Room 502, No. 95, Nong 962 Chinese Zhenguang Road, Putuo District Shanghai PRC –94– THIS DOCUMENT IS IN DRAFT FORM, INCOMPLETE AND SUBJECT TO CHANGE AND THAT THE INFORMATION MUST BE READ IN CONJUNCTION WITH THE SECTION HEADED “WARNING” ON THE COVER OF THIS DOCUMENT DIRECTORS AND PARTIES INVOLVED IN THE [REDACTED] Non-executive Directors Ms. Zhang Yitao (張藝濤) Room 301, No. 13 Building American Minghuyuan Dongfangtaiyangcheng Renhe Town Shunyi District Beijing PRC Mr. Liu Xia (劉夏) National Satellite Ocean Chinese Application Service No. 8, Dahuisi, Haidian District Beijing PRC Independent Non-executive Directors Mr. Song Ruilin (宋瑞霖) Room 202, Unit 4, Building 3 Chinese No. A28, Guangqumenwai Street Chaoyang District Beijing PRC Mr. Fei John Xiang (費翔) Room D, 22/F, Building 2A American 8 Wui Cheung Road, The Austin Tsim Sha Tsui, Kowloon Hong Kong Mr. -

Study on the Characteristics of Beijing Subsidence Based on Ps- Insar/Leveling and Primary Investigation of the Relationship with Fault Zone
The International Archives of the Photogrammetry, Remote Sensing and Spatial Information Sciences, Volume XLIII-B3-2021 XXIV ISPRS Congress (2021 edition) STUDY ON THE CHARACTERISTICS OF BEIJING SUBSIDENCE BASED ON PS- INSAR/LEVELING AND PRIMARY INVESTIGATION OF THE RELATIONSHIP WITH FAULT ZONE WANG Xiaoqing, ZHANG Peng, WANG Yongshang, SUN Zhanyi Dept of Geodesy, National Geomatics Center of China, China-(xqwang, zhangpeng, szy, wys)@ngcc.cn KEY WORDS: Beijing land subsidence, PS-InSAR, levelling, features study, fault zone. ABSTRACT: The severe land subsidence could lead to ground collapse, building damage and a series of disasters. Up to now, the land subsidence has occurred in more than 50 cities in China, which seriously affects the life and production safety of local people and restricts the development of cities. While, Beijing is one of the most serious cities. This paper takes the urban area of Beijing as an example. PS- InSAR technology is used to process 40 scenes of Terra SAR images from 2010 to 2015, and the high-coherence points are selected by fusing the two algorithms of coherence coefficient and amplitude deviation. In order to verify the reliability of the results, the second-level measurement results are compared with the PS-InSAR deformation results, and five leveling points are used to evaluate the accuracy. The results show that: the maximum absolute error between the Leveling results and the InSAR measurement result is 8.87mm, and the standard error is 3.22mm, which meets the accuracy requirements. And areas with serious subsidence occur in Changping District, Haidian District, Daxing District, and Chaoyang District; there is no obvious subsidence trend in the central and eastern parts of Dongcheng, Xicheng and Fengtai District, and the surface is relatively stable. -

United States Bankruptcy Court Northern District of Illinois Eastern Division
Case 12-27488 Doc 49 Filed 07/27/12 Entered 07/27/12 13:10:45 Desc Main Document Page 1 of 343 UNITED STATES BANKRUPTCY COURT NORTHERN DISTRICT OF ILLINOIS EASTERN DIVISION In re: ) Chapter 7 ) PEREGRINE FINANCIAL GROUP, INC., ) Case No. 12-27488 ) ) ) Honorable Judge Carol A. Doyle Debtor. ) ) Hearing Date: August 9, 2012 ) Hearing Time: 10:00 a.m. NOTICE OF MOTION TO: See Attached PLEASE TAKE NOTICE that on August 9, 2012 at 10:00 a.m., the undersigned shall appear before the Honorable Carol A. Doyle, United States Bankruptcy Judge for the United States Bankruptcy Court, Northern District of Illinois, Eastern Division, in Courtroom 742 of the Dirksen Federal Building, 219 South Dearborn Street, Chicago, Illinois 60604, and then and there present the TRUSTEE’S MOTION FOR ORDER APPROVING PROCEDURES FOR FIXING PRICING AND CLAIM AMOUNTS IN CONNECTION WITH THE TERMINATION AND LIQUIDATION OF FOREIGN EXCHANGE CUSTOMER AGREEMENTS (the “Motion”). PLEASE TAKE FURTHER NOTICE that if you are a foreign exchange customer of Peregrine Financial Group, Inc. or otherwise received this Notice, your rights may be affected by the Motion. PLEASE TAKE FURTHER NOTICE that a copy of the Motion is available on the Trustee’s website, www.PFGChapter7.com, or upon request sent to [email protected]. Respectfully submitted, Ira Bodenstein, not personally, but as chapter 7 trustee for the estate of Peregrine Financial Group, Inc. Dated: July 27, 2012 By: /s/ John Guzzardo One of his proposed attorneys Robert M. Fishman (#3124316) Salvatore Barbatano (#0109681) John Guzzardo (#6283016) Shaw Gussis Fishman Glantz {10403-001 NOM A0323583.DOC}4841-1459-7392.2 Case 12-27488 Doc 49 Filed 07/27/12 Entered 07/27/12 13:10:45 Desc Main Document Page 2 of 343 Wolfson & Towbin LLC 321 North Clark Street, Suite 800 Chicago, IL 60654 Phone: (877) 465-1849 [email protected] Proposed Counsel to the Trustee and Geoffrey S. -

The Analysis of Legal Issues to the Houses with Limited Property Rights Yilin Wang
Advances in Social Science, Education and Humanities Research, volume 101 4th International Conference on Education, Management and Computing Technology (ICEMCT 2017) The Analysis of Legal Issues to the Houses With Limited Property Rights Yilin Wang College of Humanities and Social Sciences,North China Electric Power University, Beijing, 102206, China Keyword: Jurisprudence,The houses with limited property rights,Legal issues,Legal crises,Solution Abstracts. In recent years houses with limited property rights are inevitably becoming a topic that ignites controversy on a number of fronts. What is the limited property rights for houses?How shall we give a proper definition for them? Why are they so prosperous and what is the authority’s stance?Whether they are legal or not? What legal crises could they cause?And why it’s difficult to deal with?So many questions are urgent to be solved. And in this paper,I will explain the questions of all the above all. Introduction Houses with limited property rights are buildings which are constructed by developers or collective organizations on rural land. People who are not the members in collective organizations could not possess the certificate of house property. Therefore this kind of structure was called small property rights house compared with the full property rights house. Fig. 1 Limited property rights houses in a housing estate in Changping, Beijing According to the statistics,the area of the limited property rights houses has exceeded 0.76 billion square meters in our country from 1995 to 2010, more than 8 percent of the completed residential area during that same period. -

Research Into Various Aspects of Loss Brought by Urban Traffic
6th International Conference on Electronic, Mechanical, Information and Management (EMIM 2016) Research into Various Aspects of Loss Brought by Urban Traffic Congestion and Countermeasures Zixin Song1, a*, Liyi Tian1, Chao Zhang1, Yucong Wu1 and Qian Luo1 1No.2 Beinong Road, Huilongguan Town, Changping District, Beijing, North China Electric Power University [email protected] Keywords: Traffic jams; Huilongguan; Loss; Recommendations Abstract. With the development of economy, Chinese urbanization has accelerated already, the people's living standards and quality of life have also increased steadily, and car ownership has gradually increased. Traffic congestion caused by a series of reasons including road planning and traffic control has become a big problem which cannot be ignored blocking the urbanization city. This paper chooses Huilongguan area as the research object, visiting the residents there and field research, we acquire the relevant data and then analyze environmental pollution, energy consumption and various losses which brought by the traffic congestion. Finally, provide the improvement of the relevant recommendations. Introduction Concept of Congestion. For traffic congestion, the Texas Department of transportation proposed the definition of traffic congestion: when the travel time more than in small traffic flow or free flow travel environment under normal incidence of travel time, produce larger delay of traffic state, when the delay exceeds the public to generally accepted boundaries, said unacceptable traffic congestion. [1] Research Background. In the past 20 years, our country has obtained the unprecedented development. However, the traffic jam problem in some cities is also unprecedented. According to the website of the National Bureau of Statistics announced the 2014 National Economy and Social Development Statistical Bulletin, by the end of 2014, national civilian car retains the quantity to 15447 million vehicles, up 12.4 percent over the previous year, which private car retains the quantity 125.84 million, an increase of 15.5%. -
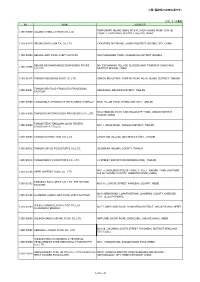
中国(偶蹄類の加熱処理肉等) 2021/5/25通知 No. NAME
中国(偶蹄類の加熱処理肉等) 2021/5/25通知 No. NAME ADDRESS TEMPORARY HUANG GANG NO.670, SHUN HUANG ROAD, SUN HE 1100/03009 BEIJING HORMEL FOODS CO.,LTD. COUNTY, CHAOYANG DISTRICT, BEIJING, CHINA 1100/03015 BEIJING DAFA CHIA TAI CO.,LTD YANGZHEN DUZHUANG, SHUNYI DISTRICT, BEIJING CITY, CHINA 1100/03039 BEIJING JIAYI FOOD JOINT FACTORY XIAOTANGSHAN TOWN, CHANGPING DISTRICT, BEIJING BEIJING ER SHANG MOQI ZHONGHONG FOODS NO. 233 NANGAO VILLAGE CUIGEZHUANG TOWNSHIP CHAOYANG 1100/15006 CO.,LTD. DISTRICT BEIJING, CHINA 1200/01025 TIANJIN LINGCHUAN FOOD CO.,LTD XINKOU INDUSTRIAL ZONE BY ROAD NO.18, XIQING DISTRICT, TIANJIN TIANJIN 3RD FOOD PRODUCTS PROCESSING 1200/03001 HANJIASHU, BEICHEN DISTRICT, TIANJIN FACTORY 1200/03008 TIANJIN MEAT PRODUCTS PROCESSING COMPLEX NO.8, YUEJIN ROAD, DONGLI DISTRICT, TIANJIN NO.8 XINWANG ROAD, SHUANGQIAOHE TOWN, JINNAN DISTRICT, 1200/03009 TIANJIN DONGTIAN FOODS PROCESSING CO., LTD TIANJIN, CHINA TIANJIN TEDA TIANQUAN QUICK FROZEN 1200/29002 NO.11, JINGU ROAD, TANGGU DISTRICT, TIANJIN FOODSTUFFS CO.,LTD. 1200/29005 TIANJIN GUOSHI FOOD CO., LTD. LANGYUAN VILLAGE, BEICHEN DISTRICT, TIANJIN 1200/29012 TIANJIN SHIYOU FOODSTUFFS CO.,LTD. SICUNDIAN, WUQING COUNTY, TIANJIN 1200/29016 TIANJIN NICKY FOODSTUFFS CO., LTD. 13 STREET, EXPORT PROCESSING ZONE, TIANJIN NO.171, MUSLIM BUSINESS TRADE STREET, XIADIAN TOWN, DACHANG 1300/03105 HEBEI SUPERB FOODS CO., LTD HUI AUTONOMY COUNTY, HEBEI PROVINCE, CHINA KANGBAO BAILU MEAT CO., LTD. THE SECOND 1300/03152 NO.134, GONGYE STREET, KANGBAO COUNTY, HEBEI FACTORY NO.9 HEBIN ROAD, LUANPINGTOWN, LUANPING COUNTY, CHENGDE 1300/03158 LUANPING HUADU JIAYI FOOD JOINT FACTORY CITY, HEBEI PROVINCE SHIJIAZHUANG DEYUAN FOOD CO.,LTD. 1300/03160 NO.77, XINYUANXI ROAD, LUANCHENG DISTRICT, SHIJIAZHUANG, HEBEI LUANCHENG BRANCH 1300/08040 SHIJIAZHUANG HUIKANG FOOD CO.,LTD.