Species-Specific Responses to Climate Change and Community Composition Determine Future Calcification Rates of Florida Keys Reefs
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Mouthpiece
Our Web Page The Mouthpiece www.activedivers. org/ May 2003 THE ACTIVE DIVERS ASSOCIATION NEWSLETTER April-May Dive Schedule Sat. 4-26 PM North Key Largo Sites may include: The Christ of the Abyss, the Elbow, N. Dry Rocks, N. N. Dry Rocks, Grecian Rocks, Carysfort, Shark Reef. Average depth 30’, usually no current. Average visibility is 30-50’ with many tropi- cals, morays, cudas. Sun. 5-4 PM Islamorada Just 4 miles past Tavernier, sites may include: Hammerhead, The Canyon, El I nfante, Crocker, No Name, The Valley Aquarium, Alligator. Average depth 30-40’, visibility 40-50’. Some current, many fish, shallow wrecks Sat. 5-10 PM Tavernier Sites may include: Conch Reef, Davis Ledge, Hens & Chickens, Little Conch, Capt. Tom’s Ledge, 40’ Ledge, Fish Trap, Horseshoe. Average depth 30’, average visibility 40-60’, many morays, schooling tropicals, un- usual pillar corals. Sat. 5-17 AM Biscayne Park (inc tanks) Sites may include: Rocky Reef, Elkhorn Forest, Ball Buoy, Far Out Reef, Cuda Ledge. Usually no currents, massive corals, small caves. The Keys “Best Kept Secret”, only one commercial boat allowed in area. Depth 20-30’, vis 30-60’. Sat. 5-24 PM Tenneco Towers (Advanced) Choice of more than 40 wrecks. Some unlike anywhere else: Jet airliner, M-60 Tanks, Tankers, Freighters, Tugs, Barges. Most are intact with penetration possible. Average depth 90’, visibility 40-50’. Expect cur- rents and dramatic profiles, many fish. See advanced criterion this issue. All Dives $35.00 New for 2003! Members may now take advantage of NEW DIVE PACS and freeze 2002 prices for this year! Look at these savings 10 packs—10 dives for only $290 Save up To $60 5 packs—5 dives for only $150 Save up to $25 You may use these pacs for any local dive except Biscayne National Park. -

Socan Monitoring Workshop
SOCAN MONITORING WORKSHOP Image credit: Lauren Valentino, NOAA/AOML 2/28/17 WorksHop Report SOCAN MONITORING WORKSHOP SOCAN MONITORING WORKSHOP IDENTIFYING PRIORITY LOCATIONS FOR OCEAN ACIDIFICATION MONITORING IN THE U.S. SOUTHEAST SUMMARY The Southeast Ocean and Coastal Acidification Network (SOCAN) held a worksHop in CHarleston, SoutH Carolina to facilitate discussion on priority locations for ocean acidification monitoring in tHe SoutHeast. The discussion included identification of key gradients in physical, chemical and biological parameters along tHe SoutHeast coast, a review of current monitoring efforts, and an assessment of stakeHolder needs. Sixteen monitoring locations were identified as potential acidification monitoring locations (see page 12). The following three monitoring locations were HigHligHted as priority sites tHat would furtHer our understanding of the chemistry and regional drivers of ocean acidification and address stakeholder needs: (1) Sapelo Island, GA (2) Gulf Stream, offsHore of Gray’s Reef, GA (3) Biscayne National Park, FL The workshop concluded witH a discussion of logistics and opportunities to pursue monitoring at tHe recommended locations. A copy of tHe agenda is included in Appendix 1. PROCEEDINGS Approximately 16 experts gatHered for tHe SOCAN Monitoring Workshop to outline recommendations for priority ocean acidification monitoring locations in tHe SoutHeast (Attendee List, Appendix 2). The worksHop began witH introductory remarks regarding tHe structure and responsibilities of SOCAN and SECOORA. Following the introductory remarks, participants reviewed the proposed agenda; no modifications were made. The first half-day was spent reviewing tHe state of ocean acidification science and regional response. Kim Yates sHared a syntHesis of tHe 2016 SOCAN State of the Science meeting, wHicH included a review of webinars and key findings related to OA chemistry, modeling and organismal response. -

Cryptic Herbivorous Invertebrates Restructure the Composition of Degraded Coral Reef Communities in the Florida Keys, Florida, USA
Old Dominion University ODU Digital Commons Biological Sciences Theses & Dissertations Biological Sciences Spring 2019 Cryptic Herbivorous Invertebrates Restructure the Composition of Degraded Coral Reef Communities in the Florida Keys, Florida, USA Angelo Jason Spadaro Old Dominion University, [email protected] Follow this and additional works at: https://digitalcommons.odu.edu/biology_etds Part of the Biology Commons, Ecology and Evolutionary Biology Commons, and the Natural Resources and Conservation Commons Recommended Citation Spadaro, Angelo J.. "Cryptic Herbivorous Invertebrates Restructure the Composition of Degraded Coral Reef Communities in the Florida Keys, Florida, USA" (2019). Doctor of Philosophy (PhD), Dissertation, Biological Sciences, Old Dominion University, DOI: 10.25777/fg35-1j72 https://digitalcommons.odu.edu/biology_etds/86 This Dissertation is brought to you for free and open access by the Biological Sciences at ODU Digital Commons. It has been accepted for inclusion in Biological Sciences Theses & Dissertations by an authorized administrator of ODU Digital Commons. For more information, please contact [email protected]. CRYPTIC HERBIVOROUS INVERTEBRATES RESTRUCTURE THE COMPOSITION OF DEGRADED CORAL REEF COMMUNITIES IN THE FLORIDA KEYS, FLORIDA, USA by Angelo Jason Spadaro B.S. May 2010, Old Dominion University A Dissertation Submitted to the Faculty of Old Dominion University in Partial Fulfillment of the Requirements for the Degree of DOCTOR OF PHILOSOPHY ECOLOGICAL SCIENCES OLD DOMINION UNIVERSITY May 2019 Approved by: Mark J Butler, IV (Director) Eric Walters (Member) Dan Barshis (Member) Seabird McKeon (Member) ABSTRACT CRYPTC HERBIVOROUS INVERTEBRATES RESTRUCTURE THE COMPOSITION OF DEGRADED CORAL REEF COMMUNITIES IN THE FLORIDA KEYS, FLORIDA, USA Angelo Jason Spadaro Old Dominion University, 2019 Director: Dr. -

An Environmental Assessment of the John Pennekamp Coral Reef State Park and the Key Largo Coral Reef Marine Sanctuary (Unpublished 1983 Report)
An environmental assessment of the John Pennekamp Coral Reef State Park and the Key Largo Coral Reef Marine Sanctuary (Unpublished 1983 Report) Item Type monograph Authors Voss, Gilbert L.; Voss, Nancy A.; Cantillo, Andriana Y.; Bello, Maria J. Publisher NOAA/National Ocean Service/National Centers for Coastal Ocean Science Download date 07/10/2021 01:47:07 Link to Item http://hdl.handle.net/1834/19992 NOAA/University of Miami Joint Publication NOAA Technical Memorandum NOS NCCOS CCMA 161 NOAA LISD Current References 2002-6 University of Miami RSMAS TR 2002-03 Coastal and Estuarine Data Archaeology and Rescue Program AN ENVIRONMENTAL ASSESSMENT OF THE JOHN PENNEKAMP CORAL REEF STATE PARK AND THE KEY LARGO CORAL REEF MARINE SANCTUARY (Unpublished 1983 Report) November 2002 US Department of Commerce University of Miami National Oceanic and Atmospheric Rosenstiel School of Marine and Administration Atmospheric Science Silver Spring, MD Miami, FL a NOAA/University of Miami Joint Publication NOAA Technical Memorandum NOS NCCOS CCMA 161 NOAA LISD Current References 2002-6 University of Miami RSMAS TR 2002-03 AN ENVIRONMENTAL ASSESSMENT OF THE JOHN PENNEKAMP CORAL REEF STATE PARK AND THE KEY LARGO CORAL REEF MARINE SANCTUARY (Unpublished 1983 Report) Gilbert L. Voss Rosenstiel School of Marine and Atmospheric Science University of Miami Nancy A. Voss Rosenstiel School of Marine and Atmospheric Science University of Miami Adriana Y. Cantillo NOAA National Ocean Service Maria J. Bello NOAA Miami Regional Library (Editors, 2002) November 2002 United States National Oceanic and Department of Commerce Atmospheric Administration National Ocean Service Donald L. Evans Conrad C. Lautenbacher, Jr. -

Reef Explorer Guide Highlights the Underwater World ALLIGATOR of the Florida Keys, Including Unique Coral Reefs from Key Largo to OLD CANNON Key West
REEF EXPLORER The Florida Keys & Key West, "come as you are" © 2018 Monroe County Tourist Development Council. All rights reserved. MCTDU-3471 • 15K • 7/18 fla-keys.com/diving GULF OF FT. JEFFERSON NATIONAL MONUMNET MEXICO AND DRY TORTUGAS (70 MILES WEST OF KEY WEST) COTTRELL KEY YELLOW WESTERN ROCKS DRY ROCKS SAND Marathon KEY COFFIN’S ROCK PATCH KEY EASTERN BIG PINE KEY & THE LOWER KEYS DRY ROCKS DELTA WESTERN SOMBRERO SHOALS SAMBOS AMERICAN PORKFISH SHOALS KISSING HERMAN’S GRUNTS LOOE KEY HOLE SAMANTHA’S NATIONAL MARINE SANCTUARY OUTER REEF CARYSFORT ELBOW DRY ROCKS CHRIST GRECIAN CHRISTOF THE ROCKS ABYSS OF THE KEY ABYSSA LARGO (ARTIFICIAL REEF) How it works FRENCH How it works PICKLES Congratulations! You are on your way to becoming a Reef Explorer — enjoying at least one of the unique diving ISLAMORADA HEN & CONCH CHICKENS REEF MOLASSES and snorkeling experiences in each region of the Florida Keys: LITTLE SPANISH CONCH Key Largo, Islamorada, Marathon, Big Pine Key & The Lower Keys PLATE FLEET and Key West. DAVIS CROCKER REEF REEF/WALL Beginners and experienced divers alike can become a Reef Explorer. This Reef Explorer Guide highlights the underwater world ALLIGATOR of the Florida Keys, including unique coral reefs from Key Largo to OLD CANNON Key West. To participate, pursue validation from any dive or snorkel PORKFISH HORSESHOE operator in each of the five regions. Upon completion of your last reef ATLANTIC exploration, email us at [email protected] to receive an access OCEAN code for a personalized Keys Reef Explorer poster with your name on it. -

Jan 2021 London Zoo Stocklist.Pdf (596.63
ZSL London Zoo - January 2021 stocklist Status at 01.01.2021 m f unk Invertebrata Aurelia aurita * Moon jellyfish 0 0 150 Pachyclavularia violacea * Purple star coral 0 0 1 Tubipora musica * Organ-pipe coral 0 0 2 Pinnigorgia sp. * Sea fan 0 0 20 Sarcophyton sp. * Leathery soft coral 0 0 5 Sinularia sp. * Leathery soft coral 0 0 18 Sinularia dura * Cabbage leather coral 0 0 4 Sinularia polydactyla * Many-fingered leather coral 0 0 3 Xenia sp. * Yellow star coral 0 0 1 Heliopora coerulea * Blue coral 0 0 12 Entacmaea quadricolor Bladdertipped anemone 0 0 1 Epicystis sp. * Speckled anemone 0 0 1 Phymanthus crucifer * Red beaded anemone 0 0 11 Heteractis sp. * Elegant armed anemone 0 0 1 Stichodactyla tapetum Mini carpet anemone 0 0 1 Discosoma sp. * Umbrella false coral 0 0 21 Rhodactis sp. * Mushroom coral 0 0 8 Ricordea sp. * Emerald false coral 0 0 19 Acropora sp. * Staghorn coral 0 0 115 Acropora humilis * Staghorn coral 0 0 1 Acropora yongei * Staghorn coral 0 0 2 Montipora sp. * Montipora coral 0 0 5 Montipora capricornis * Coral 0 0 5 Montipora confusa * Encrusting coral 0 0 22 Montipora danae * Coral 0 0 23 Montipora digitata * Finger coral 0 0 6 Montipora foliosa * Hard coral 0 0 10 Montipora hodgsoni * Coral 0 0 2 Pocillopora sp. * Cauliflower coral 0 0 27 Seriatopora hystrix * Bird nest coral 0 0 8 Stylophora sp. * Cauliflower coral 0 0 1 Stylophora pistillata * Pink cauliflower coral 0 0 23 Catalaphyllia jardinei * Elegance coral 0 0 4 Euphyllia ancora * Crescent coral 0 0 4 Euphyllia glabrescens * Joker's cap coral 0 0 2 Euphyllia paradivisa * Branching frog spawn 0 0 3 Euphyllia paraancora * Branching hammer coral 0 0 3 Euphyllia yaeyamaensis * Crescent coral 0 0 4 Plerogyra sinuosa * Bubble coral 0 0 1 Duncanopsammia axifuga + Coral 0 0 2 Tubastraea sp. -
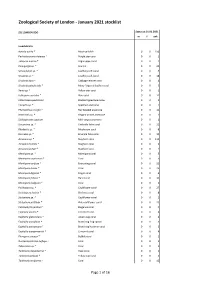
Jan 2021 ZSL Stocklist.Pdf (699.26
Zoological Society of London - January 2021 stocklist ZSL LONDON ZOO Status at 01.01.2021 m f unk Invertebrata Aurelia aurita * Moon jellyfish 0 0 150 Pachyclavularia violacea * Purple star coral 0 0 1 Tubipora musica * Organ-pipe coral 0 0 2 Pinnigorgia sp. * Sea fan 0 0 20 Sarcophyton sp. * Leathery soft coral 0 0 5 Sinularia sp. * Leathery soft coral 0 0 18 Sinularia dura * Cabbage leather coral 0 0 4 Sinularia polydactyla * Many-fingered leather coral 0 0 3 Xenia sp. * Yellow star coral 0 0 1 Heliopora coerulea * Blue coral 0 0 12 Entacmaea quadricolor Bladdertipped anemone 0 0 1 Epicystis sp. * Speckled anemone 0 0 1 Phymanthus crucifer * Red beaded anemone 0 0 11 Heteractis sp. * Elegant armed anemone 0 0 1 Stichodactyla tapetum Mini carpet anemone 0 0 1 Discosoma sp. * Umbrella false coral 0 0 21 Rhodactis sp. * Mushroom coral 0 0 8 Ricordea sp. * Emerald false coral 0 0 19 Acropora sp. * Staghorn coral 0 0 115 Acropora humilis * Staghorn coral 0 0 1 Acropora yongei * Staghorn coral 0 0 2 Montipora sp. * Montipora coral 0 0 5 Montipora capricornis * Coral 0 0 5 Montipora confusa * Encrusting coral 0 0 22 Montipora danae * Coral 0 0 23 Montipora digitata * Finger coral 0 0 6 Montipora foliosa * Hard coral 0 0 10 Montipora hodgsoni * Coral 0 0 2 Pocillopora sp. * Cauliflower coral 0 0 27 Seriatopora hystrix * Bird nest coral 0 0 8 Stylophora sp. * Cauliflower coral 0 0 1 Stylophora pistillata * Pink cauliflower coral 0 0 23 Catalaphyllia jardinei * Elegance coral 0 0 4 Euphyllia ancora * Crescent coral 0 0 4 Euphyllia glabrescens * Joker's cap coral 0 0 2 Euphyllia paradivisa * Branching frog spawn 0 0 3 Euphyllia paraancora * Branching hammer coral 0 0 3 Euphyllia yaeyamaensis * Crescent coral 0 0 4 Plerogyra sinuosa * Bubble coral 0 0 1 Duncanopsammia axifuga + Coral 0 0 2 Tubastraea sp. -

Corals Sustain Growth but Not Skeletal Density Across the Florida Keys Reef Tract Despite Ongoing Warming
bioRxiv preprint doi: https://doi.org/10.1101/310037; this version posted April 28, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Title: Corals sustain growth but not skeletal density across the Florida Keys Reef Tract despite 2 ongoing warming 3 Running head: Coral growth on the Florida Keys Reef Tract 4 5 John P. Rippe1*, Justin H. Baumann1, Daphne N. De Leener1, Hannah E. Aichelman1,y, Eric B. 6 Friedlander2, Sarah W. Davies1,g and Karl D. Castillo1,3 7 8 1Department of Marine Sciences, University of North Carolina at Chapel Hill, 3202 Murray Hall, 9 Chapel Hill, NC, USA. 10 2Department of Statistics and Operations Research, University of North Carolina at Chapel Hill, 11 318 Hanes Hall, Chapel Hill, NC, USA. 12 3Curriculum for Environment and Ecology, University of North Carolina at Chapel Hill, 3202 13 Murray Hall, Chapel Hill, NC, USA. 14 yCurrent address: Department of Biological Sciences, Old Dominion University, 110 Mills 15 Godwin Life Sciences Building, Norfolk, VA, USA. 16 gCurrent address: Department of Biology, Boston University, 5 Cummington Mall, Boston, MA, 17 USA. 18 *Corresponding author (Email: [email protected]) 19 20 Keywords: Coral reef, calcification, Caribbean, Florida Keys, sclerochronology, climate change, 21 global warming, ocean acidification 22 Paper type: Primary research article 1 bioRxiv preprint doi: https://doi.org/10.1101/310037; this version posted April 28, 2018. -

Atoll Research Bulletin No. 335 the Worldwde Coral Reef Bleaching Cycle and Related Sources of Coral Mortality by Ernest H. Will
ATOLL RESEARCH BULLETIN NO. 335 THE WORLDWDE CORAL REEF BLEACHING CYCLE AND RELATED SOURCES OF CORAL MORTALITY BY ERNEST H. WILLIAMS, JR AND LUCY BUNKLEY-WILLIAMS ISSUED BY NATIONAL MUSEUM OF NATURAL HISTORY SMITHSONIAN INSTITUTION WASHINGTON, D.C, USA. January 1990 TABLE OF CONTENTS ABSTRACT ........................................................................................................................................................1 INTRODUCTION ...........................................................................................................................................1 MATERIALS AND METHODS ..................................................................................................................2 DEFINITION OF TERMS ............................................................................................................................2 DESCRIPTION OF THE 1987-88 EVENTS BEGINNING OF THE EVENT ..........................................................................................................4 HOSTS AND NON-PHOTOSYMBIOTIC ANIMALS BLEACHED .......................................... 9 SMALL SCALE FEATURES ...............................................................................................................12 ' LARGE SCALE FEATURES ...............................................................................................................14 MORTALITIES ........................................................................................................................................21 -

Marine Debris in Reef Habitats
Marine Debris in Reef Habitats Florida Keys National Marine Sanctuary Lost Fishing Gear is Common in Sanctuary Waters Marine debris is one of the most widespread and persistent forms of pollution affecting the world’s ocean and coastal waters. Plastics, lost fishing gear, derelict vessels and other marine debris can find its way into even the most remote ocean waters where it can harm marine life. While most debris originates from activities taking place at sea, coastal communities also contribute significantly to this global threat. In the Florida Keys, where recreational and commercial fisheries have existed for over 100 years, lost fishing gear and other marine debris have accumulated on the seafloor. Lost or abandoned fishing gear and other trash entangles and harms stony corals, sea fans, sponges, sea turtles, manatees and other marine life. It also degrades seagrass, hard- bottom, coral reef and mangrove habitats and detracts from the natural beauty of the islands. For these and other reasons, citizens and resource managers of the Florida Keys National Marine Sanctuary are concerned about the environmental impacts of all marine debris. Scientists Document Prevalence of Marine Debris Coral researchers from Nova Southeastern University (NSU) Oceanographic Center (formerly with University of North Carolina Wilmington) began conducting Keys-wide surveys of marine debris in 2000 during their assessments of corals, Team OCEAN volunteers use kayaks to collect marine debris. sponges and other benthic (bottom-dwelling) marine life. Since then, debris data have been recorded in 2008, 2010-11, and 2012. The 2012 surveys were conducted in collaboration with scientists from the sanctuary, National Park Service and Florida Fish and Wildlife Conservation Commission and included 600 coral reef and hard-bottom sites from Biscayne National Park to Key West. -

Mission to Recover the Coral Reefs of the Florida Keys
MISSION: ICONIC REEFS Photo: Coral Restoration Foundation Mission to Recover the Coral Reefs of the Florida Keys he iconic coral reefs of the Florida Keys are the foundation of the vibrant regional economy that hosts 5 million visitors per year. North America’s only barrier reef protects the island communities from catastrophic storm surge, while also supporting a world-renowned destination for diving, snorkeling, Tand fishing. However, decades of compounding stress from coral bleaching, coral disease, hurricanes, and high impact human use have significantly degraded the coral reefs. The United States is on the verge of losing a national treasure. Emergency action is required to keep Florida Keys coral reefs from collapsing beyond a point at which they can be restored and protect the economy that depends on them. Restoration Strategy NOAA and partners have developed a bold mission to restore seven ecologically and culturally significant coral reefs within Florida Keys National Marine Sanctuary. The selected restoration sites represent a diversi- ty of habitats, support a range of human uses, span the full geographic range of the Florida Keys, and show a high probability of restoration success. The mission represents one of the largest investments ever under- taken in coral restoration. Informed by years of research, successful trials, and expertise from scientists and restoration practitioners, this effort complements other regional management efforts and will result in resilient and regenerative coral reefs in the Florida Keys. Carysfort Reef Horseshoe Reef Cheeca Rocks Sombrero Reef Newfound Harbor Photo: Ken Nedimyer Looe Key Reef Eastern Dry Rocks www.fisheries.noaa.gov/iconic-reefs Coral reefs are dynamic ecosystems comprised of stony corals, soft corals, sponges, and algae. -

AN ENVIRONMENTAL ASSESSMENT of the JOHN PENNEKAMP CORAL REEF STATE PARK and the KEY LARGO CORAL REEF MARINE SANCTUARY (Unpublished 1983 Report)
NOAA/University of Miami Joint Publication NOAA Technical Memorandum NOS NCCOS CCMA 161 NOAA LISD Current References 2002-6 University of Miami RSMAS TR 2002-03 Coastal and Estuarine Data Archaeology and Rescue Program AN ENVIRONMENTAL ASSESSMENT OF THE JOHN PENNEKAMP CORAL REEF STATE PARK AND THE KEY LARGO CORAL REEF MARINE SANCTUARY (Unpublished 1983 Report) November 2002 US Department of Commerce University of Miami National Oceanic and Atmospheric Rosenstiel School of Marine and Administration Atmospheric Science Silver Spring, MD Miami, FL a NOAA/University of Miami Joint Publication NOAA Technical Memorandum NOS NCCOS CCMA 161 NOAA LISD Current References 2002-6 University of Miami RSMAS TR 2002-03 AN ENVIRONMENTAL ASSESSMENT OF THE JOHN PENNEKAMP CORAL REEF STATE PARK AND THE KEY LARGO CORAL REEF MARINE SANCTUARY (Unpublished 1983 Report) Gilbert L. Voss Rosenstiel School of Marine and Atmospheric Science University of Miami Nancy A. Voss Rosenstiel School of Marine and Atmospheric Science University of Miami Adriana Y. Cantillo NOAA National Ocean Service Maria J. Bello NOAA Miami Regional Library (Editors, 2002) November 2002 United States National Oceanic and Department of Commerce Atmospheric Administration National Ocean Service Donald L. Evans Conrad C. Lautenbacher, Jr. Jamison S. Hawkins Secretary Vice-Admiral (Ret.), Acting Assistant Administrator Administrator For further information please call or write: University of Miami Rosenstiel School of Marine and Atmospheric Science 4600 Rickenbacker Cswy. Miami, FL 33149 NOAA/National Ocean Service/National Centers for Coastal Ocean Science 1305 East West Hwy. Silver Spring, MD 20910 NOAA Miami Regional Library 4301 Rickenbacker Cswy. Miami, FL 33149 Disclaimer This report has been reviewed by the National Ocean Service of the National Oceanic and Atmospheric Administration (NOAA) and approved for publication.