Biophysically Special, Unique Marine Areas of the Cook Islands
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hikurangi Plateau: Crustal Structure, Rifted Formation, and Gondwana Subduction History
Article Geochemistry 3 Volume 9, Number 7 Geophysics 3 July 2008 Q07004, doi:10.1029/2007GC001855 GeosystemsG G ISSN: 1525-2027 AN ELECTRONIC JOURNAL OF THE EARTH SCIENCES Published by AGU and the Geochemical Society Click Here for Full Article Hikurangi Plateau: Crustal structure, rifted formation, and Gondwana subduction history Bryan Davy Institute of Geological and Nuclear Sciences, P.O. Box 30368, Lower Hutt, New Zealand ([email protected]) Kaj Hoernle IFM-GEOMAR, Wischhofstraße 1-3, D-24148 Kiel, Germany Reinhard Werner Tethys Geoconsulting GmbH, Wischhofstraße 1-3, D-24148 Kiel, Germany [1] Seismic reflection profiles across the Hikurangi Plateau Large Igneous Province and adjacent margins reveal the faulted volcanic basement and overlying Mesozoic-Cenozoic sedimentary units as well as the structure of the paleoconvergent Gondwana margin at the southern plateau limit. The Hikurangi Plateau crust can be traced 50–100 km southward beneath the Chatham Rise where subduction cessation timing and geometry are interpreted to be variable along the margin. A model fit of the Hikurangi Plateau back against the Manihiki Plateau aligns the Manihiki Scarp with the eastern margin of the Rekohu Embayment. Extensional and rotated block faults which formed during the breakup of the combined Manihiki- Hikurangi plateau are interpreted in seismic sections of the Hikurangi Plateau basement. Guyots and ridge- like seamounts which are widely scattered across the Hikurangi Plateau are interpreted to have formed at 99–89 Ma immediately following Hikurangi Plateau jamming of the Gondwana convergent margin at 100 Ma. Volcanism from this period cannot be separately resolved in the seismic reflection data from basement volcanism; hence seamount formation during Manihiki-Hikurangi Plateau emplacement and breakup (125–120 Ma) cannot be ruled out. -

Diversity Visa Program, DV 2019-2021: Number of Entries During Each Online Registration Period by Region and Country of Chargeability
Diversity Visa Program, DV 2019-2021: Number of Entries During Each Online Registration Period by Region and Country of Chargeability The totals below DO NOT represent the number of diversity visas issued nor the number of selected entrants Countries marked with a "0" indicate that there were no entrants from that country or area. Countries marked with "N/A" were typically not eligible for that program year. FY 2019 FY 2020 FY 2021 Region Foreign State of Chargeabiliy Entrants Derivatives Total Entrants Derivatives Total Entrants Derivatives Total Africa Algeria 227,019 123,716 350,735 252,684 140,422 393,106 221,212 129,004 350,216 Africa Angola 17,707 25,543 43,250 14,866 20,037 34,903 14,676 18,086 32,762 Africa Benin 128,911 27,579 156,490 150,386 26,627 177,013 92,847 13,149 105,996 Africa Botswana 518 462 980 552 406 958 237 177 414 Africa Burkina Faso 37,065 7,374 44,439 30,102 5,877 35,979 6,318 2,591 8,909 Africa Burundi 20,680 16,295 36,975 22,049 19,168 41,217 12,287 11,023 23,310 Africa Cabo Verde 1,377 1,272 2,649 894 778 1,672 420 312 732 Africa Cameroon 310,373 147,979 458,352 310,802 165,676 476,478 150,970 93,151 244,121 Africa Central African Republic 1,359 893 2,252 1,242 636 1,878 906 424 1,330 Africa Chad 5,003 1,978 6,981 8,940 3,159 12,099 7,177 2,220 9,397 Africa Comoros 296 224 520 293 128 421 264 111 375 Africa Congo-Brazzaville 21,793 15,175 36,968 25,592 19,430 45,022 21,958 16,659 38,617 Africa Congo-Kinshasa 617,573 385,505 1,003,078 924,918 415,166 1,340,084 593,917 153,856 747,773 Africa Cote d'Ivoire 160,790 -

Subsidence and Growth of Pacific Cretaceous Plateaus
ELSEVIER Earth and Planetary Science Letters 161 (1998) 85±100 Subsidence and growth of Paci®c Cretaceous plateaus Garrett Ito a,Ł, Peter D. Clift b a School of Ocean and Earth Science and Technology, POST 713, University of Hawaii at Manoa, Honolulu, HI 96822, USA b Department of Geology and Geophysics, Woods Hole Oceanographic Institution, Woods Hole, MA 02543, USA Received 10 November 1997; revised version received 11 May 1998; accepted 4 June 1998 Abstract The Ontong Java, Manihiki, and Shatsky oceanic plateaus are among the Earth's largest igneous provinces and are commonly believed to have erupted rapidly during the surfacing of giant heads of initiating mantle plumes. We investigate this hypothesis by using sediment descriptions of Deep Sea Drilling Project (DSDP) and Ocean Drilling Program (ODP) drill cores to constrain plateau subsidence histories which re¯ect mantle thermal and crustal accretionary processes. We ®nd that total plateau subsidence is comparable to that expected of normal sea¯oor but less than predictions of thermal models of hotspot-affected lithosphere. If crustal emplacement was rapid, then uncertainties in paleo-water depths allow for the anomalous subsidence predicted for plumes with only moderate temperature anomalies and volumes, comparable to the sources of modern-day hotspots such as Hawaii and Iceland. Rapid emplacement over a plume head of high temperature and volume, however, is dif®cult to reconcile with the subsidence reconstructions. An alternative possibility that reconciles low subsidence over a high-temperature, high-volume plume source is a scenario in which plateau subsidence is the superposition of (1) subsidence due to the cooling of the plume source, and (2) uplift due to prolonged crustal growth in the form of magmatic underplating. -

Cook Islands of the Basicbasic Informationinformation Onon Thethe Marinemarine Resourcesresources Ofof Thethe Cookcook Islandsislands
Basic Information on the Marine Resources of the Cook Islands Basic Information on the Marine Resources of the Cook Islands Produced by the Ministry of Marine Resources Government of the Cook Islands and the Information Section Marine Resources Division Secretariat of the Pacific Community (SPC) with financial assistance from France . Acknowledgements The Ministry of Marine Resources wishes to acknowledge the following people and organisations for their contribution to the production of this Basic Information on the Marine Resources of the Cook Islands handbook: Ms Maria Clippingdale, Australian Volunteer Abroad, for compiling the information; the Cook Islands Natural Heritage Project for allowing some of its data to be used; Dr Mike King for allowing some of his drawings and illustration to be used in this handbook; Aymeric Desurmont, Secretariat of the Pacific Community (SPC) Fisheries Information Specialist, for formatting and layout and for the overall co-ordination of efforts; Kim des Rochers, SPC English Editor for editing; Jipé Le-Bars, SPC Graphic Artist, for his drawings of fish and fishing methods; Ministry of Marine Resources staff Ian Bertram, Nooroa Roi, Ben Ponia, Kori Raumea, and Joshua Mitchell for reviewing sections of this document; and, most importantly, the Government of France for its financial support. iii iv Table of Contents Introduction .................................................... 1 Tavere or taverevere ku on canoes ................................. 19 Geography ............................................................................ -

Cook Islands Priority Environmental Problems (PEC) Report: a Review and Assessment of the Priority Environmental Concerns
ISSN 1818-5614 Cook Islands priority environmental problems (PEC) report: a review and assessment of the priority environmental concerns By Island Friends Ltd. IWP-Pacific Technical Report (International Waters Project) no. 11 Global United Nations Pacific Regional Environment Development Environment Facility Programme Programme SPREP IRC Cataloguing-in-Publication Data Cook Islands priority environmental problems (PEC) report : a review and assessment of the priority environmental concerns. / [prepared by] Island Friends Ltd. – Apia, Samoa : SPREP, 2004. 106 p. ; 29 cm IWP-Pacific Technical Report (International Waters Project) no. 11 ISBN: 982-04-0274-3 ISSN: 1818-5614 1. Environmental impact analysis – Cook Islands. 2. Environmental monitoring – Cook Islands. 3. Ecological risk assessment – Cook Islands. 4. Environmental protection – Cook Islands. I. Implementation of the Strategic Action Programme of the Pacific Small Island Developing States. Project No. RAS/98/G32. III. International Waters Programme. IV. Cook Islands International Waters Programme. V. Secretariat for the Pacific Regional Environment Programme (SPREP). VI. Title. 333.714 This report was produced by SPREP’s International Waters Project that is implementing the Strategic Action Programme for the International Waters of the Pacific Small Island Developing States with funding from the Global Environment Facility. The views expressed in this report are not necessarily those of the publisher. Cover design by SPREP’s Publications Unit Editing: Ms. Talica Koroi Layout: Ms. Sasa’e Walter Printed by Marfleet Printing Co. Ltd. Apia, Samoa SPREP P O Box 240 Apia, Samoa Ph: (685) 21929 Fax: (685) 20231 Email: [email protected] Website: www.sprep.org.ws/iwp © SPREP 2004 The South Pacific Regional Environment Programme authorizes the reproduction of this material, whole or in part, provided appropriate acknowledgement is given. -

SO225 MANIHIKI II Weekly Report No
SO225 MANIHIKI II Weekly Report No. 1 R/V SONNE (19.11. – 25.11.2012) 10°13,6´S / 165°52,0´W The starting point of R/V SONNE expedition SO-225 was the port of Suva on Viti Levu island (Fiji). After 48 hours of travel the first group of scientists, engineers, and technicians from Germany arrived safe but somewhat tiered in Suva in the late evening of Saturday the 19th of November. There, the unloading of nine containers with scientific equipment for SO-225 and the mobilization of the remotely operated vehicle ROV Kiel 6000 kept us busy during the following days. In the evening of November 19th, the remaining scientists arrived in Suva, finally completing the scientific party of the SO-225 expedition. In tropical heat and occasionally heavy rain showers we managed to finish all port related cruise preparations on time thanks to the excellent support from the SONNE crew. Approximately one hour after a test program of the ROV Kiel 6000 was successfully completed, RV SONNE left Suva and headed towards the Manihiki Plateau, located ~1.000 nm to the northeast of Fiji in the area of the northern Cook Islands. Views of Suva/Fiji upon departure of R/V Sonne. RV Sonne cruises SO-224 and SO-225 are part of the cooperative project MANIHIKI II between GEOMAR and the Alfred Wegener Institute for Polar and Marine Research (AWI), funded by the German Ministry of Education and Research (BMBF). This multidisciplinary project continues previous research at the Manihiki Plateau conducted since 2007 (SO-193) on morphological, volcanological, geochemical, and geochronological studies and is now broadened by geophysical and paleoceanographic research foci. -

Monitoring the Distribution, Population Structure and Status of Sea Turtles in the Cook Islands
Monitoring the distribution, population structure and status of sea turtles in the Cook Islands Cook Islands Turtle Project: 2011 Annual Report By Dr Michael White Cook Islands Turtle Project: Annual Report 2011 www.picionline.org Research Permit: #07/09e (first issued 07/05/2009; then extended on 20/04/2010) Approved by the National Research Committee (Foundation for National Research). Partners Cook Islands Turtle Project (CITP) Pacific Islands Conservation Initiative (PICI) Ministry of Marine Resources (Pamela Maru) Pacific Divers (Proprietor: Stephen Lyon) Local Communities Cook Islands Turtle Project PO Box 1019 Titikaveka Rarotonga Cook Islands Frontispiece: Left profile of a green turtle Chelonia mydas tagged at Tongareva (2011). Photo-recognition techniques can use these facial scale patterns to confirm identity. 1 Cook Islands Turtle Project: Annual Report 2011 www.picionline.org Thanks to: Prime Minister’s Office Chief of Staff: Mac Mokoroa. Email: [email protected] Diane Charlie Tina Samson Foundation for National Research Ministry of Marine Resources Ben Ponia Pamela Maru Bill Marsters (Fishery Officer, Palmerston) Ta’angi (Fishery Officer, Manihiki) Papatu (Fishery Officer, Rakahanga) Pacific Islands Conservation Initiative URL: http://www.picionline.org Stephen Lyon & Jessica Cramp National Environment Service Vaitoti Tupa Elizabeth Munro (Biodiversity Officer) John Samuela (Former Warden of Suwarrow) Ian Karika (Scientific Advisor to Bonn Convention - CMS) Ministry of Foreign Affairs & Immigration Kave Ringi Ministry -

Atiu DRAFT Power Sector/Feasibility Report
Atiu Power Sector Feasibility Report 2004 Prepared as part of the UNDP/UNESCO Technical Assistance Project “Increase the Utilisation of Renewable Energy Technologies in the Cook Islands Energy Supply” Foreword The consultants would like to thank the many people who provided information for this report, participated in the energy survey and assisted in carrying out the energy survey. These include the Director and staff of the Energy Division who assisted in the many aspects of the field visits and data collection as well as advising on cultural and traditional protocols, the respective Island Councils, Mayors, Island Secretaries, Administrations and Aronga Mana for their kind assistance and hospitality, Government Ministries and Departments which provided assistance and the people of Atiu, Mauke and Mitiaro for their warmness and generosity whilst visiting their communities. However, the contents are the responsibility of the undersigned and do not necessarily represent the views of the Government of the Cook Islands (national as well as local), UNESCO, UNDP, or the many individuals who kindly provided information on which the study is based. Bruce Clay Herb Wade October 2004 ii ACRONYMS and ABBREVIATIONS A Amp a.g.l. Above ground level a.s.l. Above sea level AAGR Average Annual Growth Rate ABC Arial Bundled Cable AC Alternating Current ACP African Caribbean Pacific Countries ADB Asian Development Bank AIC Atiu Island Council Al Aluminium APS Atiu Power Supply CEO Chief Executive Officer COE Cost of Energy DSM Demand Side Management EEZ -
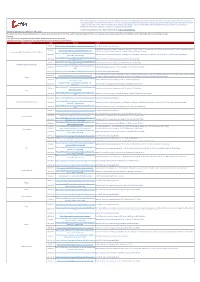
Shipping Operations Updated 6 April 2021
Please note, although we endeavour to provide you with the most up to date information derived from various third parties and sources, we cannot be held accountable for any inaccuracies or changes to this information. Inclusion of company information in this matrix does not imply any business relationship between the supplier and WFP / Logistics Cluster, and is used solely as a determinant of services, and capacities. Logistics Cluster /WFP maintain complete impartiality and are not in a position to endorse, comment on any company's suitability as a reputable service provider. If you have any updates to share, please email them to: [email protected] Shipping Operations Updated 6 April 2021 This bulletin is compiled to give all stakeholders an overview of the current impact of COVID-19 on Pacific shipping activities. It draws on sources from government, commercial and humanitarian sectors. The bulletin will now be circulated monthly. Overview • UN Bodies call for seafarers and aviation workers to be given priority access to vaccines • Massive congestion in European and Asian ports anticipated due to resolved Suez Canal blockage State / Territory Date Source Details 26-Mar-21 https://info.swireshipping.com/shipping-schedules/oceania See link for Swire Shipping schedule. Kaimana Hila arrives on 4 March. Maunawili arrives on 11 March. Daniel K. Inouye arrives on 18 March. Manukai arrives 25 March. Maunawili departs 25-Feb-21 https://www.matson.com/fss/reports/guam_s.pdf Commonwealth of the Northern Mariana Islands on 9 March. Daniel K. Inouye departs on 14 March. Manukai departs 21 March. https://www.pdl123.co.nz/common/downloads/images/sch 24-Feb-21 Neptune Pacific has vessels arriving on 7 & 21 March, 10 & 21 April, 5 & 24 May, 4 & 18 June, 7 & 18 July, 20 & 31 August and 14 September. -

Cook Islands Seabed Minerals : a Precautionary Approach to Mining / Gerald Mccormack
Cook Islands Seabed Minerals a precautionary approach to mining Gerald McCormack second edition, with corrections Cook Islands Natural Heritage Trust Rarotonga 2016 1° = primary 2° = secondary dt = dry tonnes M = million Ma = million years (from megaannus) for dates and duration mbsl = metres below sea level Mdt/y = million dry tonnes per year M/y = million per year ppm = parts per million t = tonne = 1,000kg (a.k.a. metric ton with symbol mt) wt = wet tonnes AABW = Antarctic Bottom Water BPA = Biodiversity Preservation Area CBD = Convention on Biological Diversity CISWF = Cook Islands Sovereign Wealth Fund CCD = Carbonate Compensation Depth CCZ = Clarion-Clipperton Zone DSC = Deep Sound Channel EEZ = Exclusive Economic Zone EIA - Environmental Impact Assessment GDP = Gross Domestic Product REY = Rare Earth Elements + Yttrium SBMA = Cook Islands Seabed Minerals Authority SMS = Seabed Massive Sulphides SPB = South Penrhyn Basin ISA = International Seabed Authority, a UN agency WCPFC = Western and Central Pacific Fisheries Commission Front cover An oblique view of the Cook Islands seafloor with the South Penrhyn Basin and its nodule fields in the foreground and the Manihiki Plateau in the background. Cook Islands Seabed Minerals a precautionary approach to mining Gerald McCormack second edition, with corrections Cook Islands Natural Heritage Trust Rarotonga 2016 Text and illustrations are licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. This means they are free for non-commercial use provided the author and illustrator are acknowledged. For other uses please contact the Cook Islands Natural Heritage Trust. The author extends his appreciation to the Minister of Natural Heritage, the Hon. Kiriau Turepu and to the Trust Chair, Ian Karika and his Board for their encouragement and support. -

Tuhinga Pdf for TPP:Layout 1
Tuhinga 21: 99–123 Copyright © Museum of New Zealand Te Papa Tongarewa (2010) Rediscovering the collection: Cook Islands material culture in the Museum of New Zealand Te Papa Tongarewa Grace Hutton*, Safua Akeli** and Sean Mallon*** * Museum of New Zealand Te Papa Tongarewa, PO Box 467, Wellington, New Zealand ([email protected]) ** Museum of New Zealand Te Papa Tongarewa, PO Box 467, Wellington, New Zealand ([email protected]) *** Museum of New Zealand Te Papa Tongarewa, PO Box 467, Wellington, New Zealand ([email protected]) ABSTRACT: Artefacts from the Cook Islands have been collected since the Museum of New Zealand Te Papa Tongarewa (Te Papa) opened in 1865 as the Colonial Museum. In this article we provide a historical overview of the Cook Islands collection at Te Papa. We discuss the strengths and weaknesses of the collection, review some of the factors influencing its growth, and consider the possibilities for future collection development. This article is an output of a survey of the Cook Islands collection carried out between 2007 and 2009. KEYWORDS: Te Papa, Cook Islands collection, Pacific Cultures collection, Pacific Islanders, New Zealand, museums. Introduction to New Zealand in recent decades. What began in the It is only since 1993 that the Museum of New Zealand Te nineteenth century as a comparative collection of ethno - Papa Tongarewa (Te Papa) has managed its Pacific treasures graphic ‘specimens’ – objects collected during the scientific as a separate collection. For most of the institution’s history study of peoples and cultures – has broadened to include (as the Colonial Museum from 1865 to 1907, the Dominion contemporary works by known artists. -

Commission on the Limits of the Continental Shelf in Regard to the Submission Made by the Cook Islands in Respect of the Manihiki Plateau on 16 April 20091
United Nations Convention on the Law of the Sea ____________________________________________________________ Commission on the Limits of the Continental Shelf SUMMARY OF RECOMMENDATIONS OF THE COMMISSION ON THE LIMITS OF THE CONTINENTAL SHELF IN REGARD TO THE SUBMISSION MADE BY THE COOK ISLANDS IN RESPECT OF THE MANIHIKI PLATEAU 1 ON 16 APRIL 2009 Recommendations prepared by the Subcommission established for the consideration of the Submission made by the Cook Islands Approved by the Subcommission on 31 July 2015 Approved by the Commission, with amendments, on 19 August 2016 1 The aim of this Summary is to provide information which is not of confidential or proprietary nature in order to facilitate the function of the Secretary-General in accordance with Rule 11.3 of annex III to the Rules of Procedure of the Commission (CLCS/40/Rev.1). This Summary is based on excerpts of the Recommendations and may refer to material not necessarily included either in the full Recommendations or this Summary. TABLE OF CONTENTS GLOSSARY OF TERMS ...................................................................................................................... III I. INTRODUCTION ........................................................................................................................... 1 II. CONTENTS OF THE SUBMISSION .............................................................................................. 4 A. Original Submission .................................................................................................................