1 Depth of the Vadose Zone Controls Aquifer Biogeochemical Conditions and Extent of Anthropogenic Nitrogen Removal Szymczycha B
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Vadose Water
Nimmo J R. (2009) Vadose Water. In: GeneAuthor's E. Likens, personal (Editor) copy Encyclopedia of Inland Waters. Volume 1, pp. 766-777 Oxford: Elsevier. Vadose Water J R Nimmo, U.S. Geological Survey, Menlo Park, CA, USA Published by Elsevier Inc. Introduction the porous medium in an oven until the weight is constant, and then calculate how much water was The term vadose is derived from the Latin vadosus, in the soil from the difference between the dry weight meaning ‘shallow.’ In this sense, however, it refers to and the initial wet weight. Other methods in wide- shallow depths beneath the land surface, not shallow spread use have advantages, such as being less disrup- portions of surface water bodies. The vadose zone is tive. Neutron scattering is commonly used for frequently called the unsaturated zone, and some- monitoring y as a function of depth in the field. This times the zone of aeration, as its pore space usually method is based on the high effectiveness of water, contains air as well as water. The vadose zone extends among the various components of the wet soil, in from the land surface to the water table (the lowest slowing neutrons. Commercially available equipment water table if there is more than one). has a neutron source and detector housed in a cylin- Prediction of the transport rates of water and other drical probe that can be lowered to various depths in substances within the vadose zone is critical to infil- a lined hole. Another way to monitor y in the lab or tration, runoff, erosion, plant growth, microbiota, field is by measurement of the dielectric constant of contaminant transport, aquifer recharge, and dis- the medium, usually by time-domain reflectometry charge to surface water. -

Unsaturated Zone Flow Processes, in Anderson, M.G., and Bear, J., Eds., Encyclopedia of Hydrological Sciences: Part 13--Groundwater: Chichester, UK, Wiley, V
Nimmo, J.R., 2005, Unsaturated Zone Flow Processes, in Anderson, M.G., and Bear, J., eds., Encyclopedia of Hydrological Sciences: Part 13--Groundwater: Chichester, UK, Wiley, v. 4, p. 2299-2322, doi:10.1002/0470848944.hsa161, http://www.mrw.interscience.wiley.com/ehs/articles/hsa161/frame.html. 150: Unsaturated Zone Flow Processes JOHN R NIMMO United States Geological Survey, Menlo Park, CA, US Water flow in the unsaturated zone is greatly influenced by unsaturated hydrostatics (water content, energy, pressure, and retention) and by unsaturated hydrodynamics (diffuse flow and preferential flow). Important multiphase processes include the transport of gases, nonaqueous liquids, and solid particles. Numerous means are available for determination of unsaturated conditions and properties, both measurement (of moisture state, water retention, and dynamic characteristics) and through various formulas and models that are mostly empirical in nature, but in some cases incorporating insight into unsaturated-zone physical processes. Applications to practical problems include models and techniques relating to distributions of water and energy, fluxes at the land surface, inputs, outputs, and fluxes within the unsaturated zone, all of which are frequently complicated by heterogeneity and preferential flow. Further scientific advance requires new measurement techniques and theoretical constructs that more adequately represent the important physical processes within practical modeling schemes. INTRODUCTION flow. The next section presents techniques for obtaining quantitative values of properties that influence unsaturated The unsaturated zone, sometimes called the vadose zone or flow, by direct measurement and by indirect means. The zone of aeration, plays several critical hydrologic roles. As third major section describes some of the main hydrologic a storage medium, it is a zone in which water is immediately applications related to flow in the unsaturated zone. -

Evaluating Contaminant Flux from the Vadose Zone to the Groundwater in the Hanford Central Plateau: SX Tank Farm Case Study
PNNL-23737 RPT-DVZ-AFRI-026 Evaluating Contaminant Flux from the Vadose Zone to the Groundwater in the Hanford Central Plateau: SX Tank Farm Case Study September 2015 M.J. Truex M. Oostrom G.V. Last C.E. Strickland G.D. Tartakovsky PNNL-23737 RPT-DVZ-AFRI-026 Evaluating Contaminant Flux from the Vadose Zone to the Groundwater in the Hanford Central Plateau: SX Tank Farm Case Study M.J. Truex M. Oostrom G.V. Last C.E. Strickland G.D. Tartakovsky September 2015 Prepared for the U.S. Department of Energy under Contract DE-AC05-76RL01830 Pacific Northwest National Laboratory Richland, Washington 99352 Executive Summary At the U.S. Department of Energy’s (DOE’s) Hanford Site, contaminants were discharged to the subsurface through engineered waste sites in the Hanford Central Plateau. Additional waste was released through waste storage tank leaks. Much of the contaminant inventory is still present within the unsaturated vadose zone sediments. The nature and extent of future groundwater contaminant plumes and the growth or decline of current groundwater plumes beneath the Hanford Central Plateau are a function of the contaminant flux from the vadose zone to the groundwater. In general, contaminant transport is slow through the vadose zone and it is difficult to directly measure contaminant flux in the vadose zone. Predictive analysis, supported by site characterization and monitoring data, was applied using a structured, systems-based approach to estimate the future contaminant flux to groundwater in support of remediation decisions for the vadose zone and groundwater (Truex and Carroll 2013). The SX Tank Farm was used as a case study because of the existing contaminant inventory in the vadose zone, observations of elevated moisture content in portions of the vadose zone, presence of a limited-extent groundwater plume, and the relatively large amount and wide variety of data available for the site. -

Influence of the Vadose Zone on Groundwater Pollution- a Review DOI:10
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Sri Shakthi SIET Journals /ijceae1916 ARTICLE 34256 REVIEW REVIEW Influence of the vadose zone on groundwater pollution- A review DOI:10. Banajarani Panda a, S. Chidambaram b* a Department of Earth Sciences, Annamalai University, Tamilnadu, India -608002. b Research Scientist, Water Research Centre, Kuwait Institute for Scientific Research, Safat, Kuwait. *Corresponding Author ABSTRACT: The vadose zone is the geologic profile that lies between the water table and sabarathinam.chidambaram@ the ground surface. It has low water content relative to the saturated zone and commonly gmail.com referred as the unsaturated zone. Recharge to the water table passes through the vadose (S. Chidambaram) zone and understanding transport through this region is critical in groundwater pollution studies. Groundwater pollution is controlled by a number of physical and chemical Received : 26-03-2019 processes which may retard or transform contaminants as they pass through the vadose Accepted : 05-05-2019 zone. Porous materials hold water under tension as a component of soil structure, ambient fluid pressures and other factors. When vadose zone water content is below saturation, leakage liquid as well as the dissolved materials passed on in it are retained. Hydrologically, the depth of unsaturated zone plays an important role in controlling water movement and contaminant transport from the land surface to the aquifer. The purpose of this study is to present an overview of the principles of fluid flow and moisture retention in the vadose zone and its influence on groundwater pollution. The study is presented in two parts: Part I includes descriptions of zones of soil moisture, basic principles of properties controlling the fluid distribution in pore spaces and how subsurface soil properties can be used to assess the leachate mobility. -

A National Roadmap for Vadose Zone Science & Technology
DOE/ID-10871 A National Roadmap for Vadose Zone Science & Technology Understanding, Monitoring, and Predicting Contaminant Fate and Transport in the Unsaturated Zone Revision 0.0 August 2001 A National Roadmap for Vadose Zone Science and Technology PREFACE The project management and logistical aspects of initiating a first national roadmap for vadose zone science and technology are daunting. The initial challenge was to select and assemble a team of experts who could identify the research and development needed over the next quarter century to adequately assess and predict the fate and transport of contaminants in the vadose zone. The second challenge was to ensure that the group was well-versed in U.S. Department of Energy (DOE) needs, the vadose zone cleanup efforts of other federal agencies, and the research underway in universities, the private sector, and government facilities (including the DOE national laboratories). Third, the interests and requirements of federal, state and local governments had to be considered. After a lengthy search, 10 internationally recognized individuals were selected and accepted positions on the Vadose Zone Roadmap Executive Committee. Four of these individuals also accepted the challenge to serve as workgroup chairs and to assemble the remainder of the participants for the workgroups. The full team consisted of 62 individuals, many of whom are internationally recognized vadose zone experts. These individuals (a complete list is provided in Appendix A) also provided representation from across DOE, other federal agencies, industry, academia, and the international community. In March 2000, the Executive Committee met to begin its task. Over the next year, this committee and four (ultimately condensed to three) roadmap workgroups formed the multi- disciplinary team that has outlined in these pages a set of vadose zone research and infrastructure priorities for the next quarter-century. -

Role of Vadose-Zone Flow Processes in Regional-Scale Hydrology: Review, Opportunities and Challenges
6 Role of vadose-zone flow processes in regional-scale hydrology: review, opportunities and challenges Thomas Harter and Jan W. Hopmans Abstract At the regional scale, vadose-zone processes are recognized for controlling both short-term dynamics in watershed hydrology and long-term water balances of hydrologic basins. In this paper we explore the various conceptual and mathematical models that have been proposed or could be considered to represent water fluxes in the vadose zone at the catchment, watershed or regional scale. Such models have been in existence in two largely disconnected disciplines: on the one hand, watershed hydrologists and, more recently, climate modelers frequently conceptualize the vadose zone as a zero-dimensional black box represented by lumped parameter models. On the other hand, soil physicists, equipped with tools to measure system and system- state properties directly in the vadose zone at scales of 10-2 – 100 m, have relied on Richards’ equation, a physically based, fully parameterized four-dimensional space– time model to represent unsaturated flow at the laboratory or field-plot scale. Over the past thirty years, the modeling efforts of the two disciplines have increasingly converged: hydrologists downscale their models by employing distributed (rather than lumped) models of varying complexity, while soil physicists have employed stochastic methods to upscale from their local-scale measurements and their local- scale physical understanding of flow processes to the field and regional scale. The lead question in this work is how the typical small-scale vadose-zone measurements relate to the large-scale representative or ‘effective’ parameter values of variously complex regional vadose-zone models. -

Vadose Zone Lag Time Effect on Groundwater Drought in A
water Article Vadose Zone Lag Time Effect on Groundwater Drought in a Temperate Climate Buruk Kitachew Wossenyeleh 1,* , Boud Verbeiren 2 , Jan Diels 1 and Marijke Huysmans 1,2 1 Department of Earth and Environmental Sciences, KU Leuven, Celestijnenlaan 200E, BE-3001 Leuven, Belgium; [email protected] (J.D.); [email protected] or [email protected] (M.H.) 2 Department of Hydrology and Hydraulic Engineering, Vrije Universiteit Brussel, Pleinlaan 2, BE-1050 Brussels, Belgium; [email protected] * Correspondence: [email protected] or [email protected] Received: 6 June 2020; Accepted: 23 July 2020; Published: 26 July 2020 Abstract: An essential factor in the propagation of drought, from meteorological drought to groundwater drought, is the delay between a precipitation event and the groundwater recharge reaching the groundwater table. This delay, which mainly occurs in the vadose zone of the hydrological cycle, is often poorly studied. Therefore, this paper proposes a method for estimating the spatially distributed delay in the vadose zone using the kinematic wave approximation of Richards’ equation combined with the van Genuchten–Burdine and Brooks–Corey parametric model. The modeling was approached (1) using a detailed parametrization of soil and geological layers and (2) using lumped hydraulic and physical properties of geological layers. The results of both approaches were compared against the physically based flow model Hydrus-1D. This analysis shows that using a detailed parametrization of soil and geological layers results in good comparison, with a Nash–Sutcliffe efficiency of 0.89 for Brooks–Corey and 0.80 for van Genuchten–Burdine. The delay result of the Brooks–Corey model was incorporated into the groundwater recharge time series from 1980 to 2013 to analyze the effect of this delay on groundwater drought. -

Vadose Zone Characterization and Monitoring Current Technologies, Applications, and Future Developments
3 Vadose Zone Characterization and Monitoring Current Technologies, Applications, and Future Developments Boris Faybishenko Contributors: M. Bandurraga, M. Conrad, P. Cook, C. Eddy-Dilek, L. Everett, FRx Inc. of Cincinnati, T. Hazen, S. Hubbard, A.R. Hutter, P. Jordan, C. Keller, F.J. Leij, N. Loaiciga, E.L. Majer, L. Murdoch, S. Renehan, B. Riha, J. Rossabi, Y. Rubin, A. Simmons, S. Weeks, C.V. Williams INTRODUCTION NEEDS FOR VADOSE ZONE CHARACTERIZATION AND MONITORING Vadose zone characterization and monitoring are essential for: • Development of a complete and accurate assessment of the inven- tory, distribution, and movement of contaminants in unsaturated- saturated soils and rocks. • Development of improved predictive methods for liquid flow and contaminant transport. • Design of remediation systems (barrier systems, stabilization of buried wastes in situ, cover systems for waste isolation, in situ treat- ment barriers of dispersed contaminant plumes, bioreactive treat- ment methods of organic solvents in sediments and groundwater). • Design of chemical treatment technologies to destroy or immobilize highly concentrated contaminant sources (metals, radionuclides, explosive residues, and solvents) accumulated in the subsurface. 133 134 VADOSE ZONE SCIENCE AND TECHNOLOGY SOLUTIONS Development of appropriate conceptual models of water flow and chemical transport in the vadose zone soil-rock formation is critical for developing adequate predictive modeling methods and designing cost- effective remediation techniques. These conceptual models of unsatu- rated heterogeneous soils must take into account the processes of preferential and fast water seepage and contaminant transport toward the underlying aquifer. Such processes are enhanced under episodic natural precipitation, snowmelt, and extreme chemistry of waste leaks from tanks, cribs, and other surface sources. -

Characterizing Soils for Hazardous Waste Site Assessments
United States Office of Office of Solid EPA/540/4-91/003 Environmental Protection Research and Waste March 1991 Agency Development and Emergency Response Ground-Water Issue CHARACTERIZING SOILS FOR HAZARDOUS WASTE SITE ASSESSMENTS R. P. Breckenridgel, J. R. Williams2, and J. F. Keckl INTRODUCTION tion provides data types required for decision The Regional Superfund Ground Water making in three distinct RI/FS tasks: Forum is a group of ground-water scentists representing EPA’s Regional Offices, orga- 1.. Determination of the nature and extent of nized to exchange up-to-date information re- soil contamination. lated to ground-water remediation at hazard- ous waste sites. Soil characterization at 2. Risk assessment, and determination of hazardous waste sites is an issue identified by risk-baaed soil dean-up levels. the forum as a concern of CERCLA decision- makers. 3. Determination of the potential effective- ness of soil remediation alternatives. To address this issue, this paper was pre- pared through support from EMSL-LV and Identification of data types required for the first RSKERL, under the direction of R. P. task, determination of the nature and extent of Breckenridge, with the support of the contamination, is relatively straight forward. Superfund Technical Support Project For The nature of contamination is related to the further information contact Ken Brown, EMSL- types of operations conducted at the site. LV Center Director, at FTS 545-2270 or R. P. Existing records, if available, and interviews Breckenridge at FTS 583-0757. with personnel familiar with the site history are good sources of information to help determine Site investigation and remediation under the the types of contaminants potentially present. -

Package 'Soilhyp'
Package ‘SoilHyP’ January 8, 2021 Type Package Title Soil Hydraulic Properties Version 0.1.5 Date 2021-01-08 Maintainer Ullrich Dettmann <[email protected]> Description Provides functions for (1) soil water retention (SWC) and unsaturated hydraulic conduc- tivity (Ku) (van Genuchten-Mualem (vGM or vG) [1, 2], Peters-Durner- Iden (PDI) [3, 4, 5], Brooks and Corey (bc) [8]), (2) fitting of parameter for SWC and/or Ku us- ing Shuffled Complex Evolution (SCE) optimisation and (3) calculation of soil hydraulic proper- ties (Ku and soil water contents) based on the simplified evaporation method (SEM) [6, 7]. Main references: [1] van Genuchten (1980) <doi:10.2136/sssaj1980.03615995004400050002x>, [2] Mualem (1976) <doi:10.1029/WR012i003p00513>, [3] Peters (2013) <doi:10.1002/wrcr.20548>, [4] Iden and Durner (2013) <doi:10.1002/2014WR015937>, [5] Peters (2014) <doi:10.1002/2014WR015937>, [6] Wind G. P. (1966), [7] Peters and Durner (2008) <doi:10.1016/j.jhydrol.2008.04.016> and [8] Brooks and Corey (1964). Imports data.table (>= 1.13), lubridate (>= 1.7.9) BugReports https://bitbucket.org/UlliD/soilhyp/issues Encoding UTF-8 License GPL (>= 2) Depends R (>= 4.0.0) RoxygenNote 7.1.1 NeedsCompilation no Author Ullrich Dettmann [aut, cre], Felix Andrews [ctb] (For the code copied from the hydromad::SCEoptim function (Version: 0.9-15) which is not on r-cran (https://github.com/floybix/hydromad). The SCEoptim function is adapted, and substantially revised from Brecht Donckels MATLAB code which is in turn adopted from Qingyun Duans MATLAB code), 1 2 AIC_HY Brecht Donckels [ctb] (For the Matlab code which was adapted and reviesed in the hydromad::SCEoptim function.), Qingyun Duan [ctb] (For the MATLAB code adapted from Brecht Donkels.) Repository CRAN Date/Publication 2021-01-08 16:00:06 UTC R topics documented: AIC_HY . -
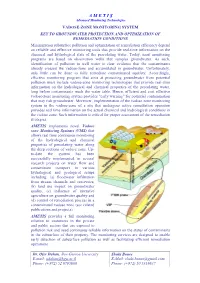
Vadose-Zone Monitoring System: Key to Groundwater Protection and Optimization of Remediation Conditions
A M E T I S Advanced Monitoring Technologies VADOSE-ZONE MONITORING SYSTEM KEY TO GROUNDWATER PROTECTION AND OPTIMIZATION OF REMEDIATION CONDITIONS Minimization subsurface pollution and optimization of remediation efficiency depend on reliable and effective monitoring tools that provide real-time information on the chemical and hydrological state of the percolating water. Today, most monitoring programs are based on observation wells that samples groundwater. As such, identification of pollution in well water is clear evidence that the contaminants already crossed the vadose-zone and accumulated in groundwater. Unfortunately, only little can be done to fully remediate contaminated aquifers. Accordingly, effective monitoring program that aims at protecting groundwater from potential pollution must include vadose-zone monitoring technologies that provide real-time information on the hydrological and chemical properties of the percolating water, long before contaminants reach the water table. Hence, efficient and cost effective vadose-zone monitoring system provides "early warning" for potential contamination that may risk groundwater. Moreover, implementation of the vadose zone monitoring system in the vadose-zone of a site that undergoes active remediation operation provides real time information on the actual chemical and hydrological conditions in the vadose zone. Such information is critical for proper assessment of the remediation strategies. AMETIS implements novel Vadose zone Monitoring Systems (VMS) that allows real time continuous -

Effects of Shallow Groundwater Management on the Spatial and Temporal Variability of Boron and Salinity in an Irrigated Field
Effects of Shallow Groundwater Management on the Spatial and Temporal Variability of Boron and Salinity in an Irrigated Field P. J. Shouse,* S. Goldberg, T. H. Skaggs, R. W. O. Soppe, and J. E. Ayars ABSTRACT Historically, agricultural drainage water in the west- In some irrigated regions, the disposal of agricultural drainage ern USA has been discharged into surface waters, waters poses significant environmental challenges. Efforts are under- mainly river systems (Schoups et al., 2005). During the way to develop irrigation water management practices that reduce the 1970s and 1980s, drainage water in the San Joaquin volume of drainage generated. One such management strategy in- Valley was used to replenish wetlands and other wildlife volves restricting flow in subsurface drains in an effort to raise the habitats (Schoups et al., 2005). Drainage waters in San water table and induce the consumption of groundwater by crops. A Joaquin Valley carry significant concentrations of Se, As, potential complication with this management approach is that upward B, and other trace elements (Letey et al., 2002). These groundwater flow may salinize the soil and increase concentrations of potentially toxic constituents come from salts that occur phytotoxic elements such as B. In this study, salinity and B concen- naturally in the region’s soils, which were derived from trations were monitored for 3 yr in a 60-ha agricultural field located in San Joaquin Valley, California. The irrigated field was managed marine sediments. Irrigation dissolves and leaches these according to a restricted drainage, shallow groundwater management native salts. In habitats receiving drainage eventually it technique.