Nitric Oxide & Mouth Breathing
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nitric Oxide in Health and Disease of the Nervous System H-Y Yun1,2, VL Dawson1,3,4 and TM Dawson1,3
Molecular Psychiatry (1997) 2, 300–310 1997 Stockton Press All rights reserved 1359–4184/97 $12.00 PROGRESS Nitric oxide in health and disease of the nervous system H-Y Yun1,2, VL Dawson1,3,4 and TM Dawson1,3 Departments of 1Neurology; 3Neuroscience; 4Physiology, Johns Hopkins University School of Medicine, Baltimore, MD, USA Nitric oxide (NO) is a widespread and multifunctional biological messenger molecule. It mediates vasodilation of blood vessels, host defence against infectious agents and tumors, and neurotransmission of the central and peripheral nervous systems. In the nervous system, NO is generated by three nitric oxide synthase (NOS) isoforms (neuronal, endothelial and immunologic NOS). Endothelial NOS and neuronal NOS are constitutively expressed and acti- vated by elevated intracellular calcium, whereas immunologic NOS is inducible with new RNA and protein synthesis upon immune stimulation. Neuronal NOS can be transcriptionally induced under conditions such as neuronal development and injury. NO may play a role not only in physiologic neuronal functions such as neurotransmitter release, neural development, regeneration, synaptic plasticity and regulation of gene expression but also in a variety of neurological disorders in which excessive production of NO leads to neural injury. Keywords: nitric oxide synthase; endothelium-derived relaxing factor; neurotransmission; neurotoxic- ity; neurological diseases Nitric oxide is probably the smallest and most versatile NO synthases isoforms and regulation of NO bioactive molecule identified. Convergence of multi- generation disciplinary efforts in the field of immunology, cardio- vascular pharmacology, chemistry, toxicology and neu- NO is formed by the enzymatic conversion of the guan- robiology led to the revolutionary novel concept of NO idino nitrogen of l-arginine by NO synthase (NOS). -

Adverse Effects of Mouth Breathing
AGD - Academy of General Denstry hp://www.agd.org/publicaons/arcles/?ArtID=6850 Mouth breathing: Adverse effects on facial growth, health, Contact Us academics, and behavior Send to a Friend By Yosh Jefferson, DMD, MAGD Send to Printer Featured in General Dentistry , January/February 2010 Pg. 18-25 Close Window Posted on Friday, January 08, 2010 The vast majority of health care professionals are unaware of the negative impact of upper airway obstruction (mouth breathing) on normal facial growth and physiologic health. Children whose mouth breathing is untreated may develop long, narrow faces, narrow mouths, high palatal vaults, dental malocclusion, gummy smiles, and many other unattractive facial features, such as skeletal Class II or Class III facial profiles. These children do not sleep well at night due to obstructed airways; this lack of sleep can adversely affect their growth and academic performance. Many of these children are misdiagnosed with attention deficit disorder (ADD) and hyperactivity. It is important for the entire health care community (including general and pediatric dentists) to screen and diagnose for mouth breathing in adults and in children as young as 5 years of age. If mouth breathing is treated early, its negative effect on facial and dental development and the medical and social problems associated with it can be reduced or averted. Received: February 11, 2009 Accepted: May 5, 2009 The importance of facial appearances in contemporary society is undeniable. Many studies have shown that individuals with attractive facial features are more readily accepted than those with unattractive facial features, providing them with significant advantages. 1-6 However, many health care professionals (as well as the public) feel that individual facial features are the result of genetics and therefore cannot be altered or changed—in other words, the genotype ultimately controls the phenotype. -

Association Between Oral Habits, Mouth Breathing And
Association between oral habits, mouth breathing E.G. Paolantonio, N. Ludovici, and malocclusion in Italian S. Saccomanno, G. La Torre*, C. Grippaudo preschoolers Dental and Maxillofacial Institute, Head and Neck Department, Fondazione Policlinico Gemelli IRCCS, Catholic University of Sacred Heart, Rome, Italy *Department of Public Health and Infectious Diseases, Sapienza University of Rome, Italy e-mail: [email protected] DOI 10.23804/ejpd.2019.20.03.07 Abstract Introduction Etiopathogenesis of malocclusion involves not only genetic Aim This cross-sectional study was carried out to evaluate but also environmental factors, since craniofacial development the prevalence of malocclusion and associated factors in is stimulated by functional activities such as breathing, preschoolers with the aim of assessing the existence of an chewing, sucking and swallowing [Salone et al., 2013]. association between bad habits and mouth breathing with Non-nutritive sucking habits and mouth breathing are the the most severe malocclusions. most significant environmental risk factors for malocclusion Materials and methods A sample of 1616 children aged [Grippaudo et al., 2016; Gòis et al., 2008; Primoži et al., 2013], 3–6 years was visited by applying the Baby ROMA index, an as they can interfere with occlusion and normal craniofacial orthodontic treatment need index for preschool age. The development. Infants have an inherent, biological drive following were searched: the prevalence of malocclusion, for sucking, that can be satisfied through nutritive sucking, the association of bad habits and mouth breathing with including breast- and bottle-feeding, or through non-nutritive malocclusion, how often are found in association and how sucking on objects such as digits, pacifiers, or toys that may this association is statistically significant. -

Ocean Storage
277 6 Ocean storage Coordinating Lead Authors Ken Caldeira (United States), Makoto Akai (Japan) Lead Authors Peter Brewer (United States), Baixin Chen (China), Peter Haugan (Norway), Toru Iwama (Japan), Paul Johnston (United Kingdom), Haroon Kheshgi (United States), Qingquan Li (China), Takashi Ohsumi (Japan), Hans Pörtner (Germany), Chris Sabine (United States), Yoshihisa Shirayama (Japan), Jolyon Thomson (United Kingdom) Contributing Authors Jim Barry (United States), Lara Hansen (United States) Review Editors Brad De Young (Canada), Fortunat Joos (Switzerland) 278 IPCC Special Report on Carbon dioxide Capture and Storage Contents EXECUTIVE SUMMARY 279 6.7 Environmental impacts, risks, and risk management 298 6.1 Introduction and background 279 6.7.1 Introduction to biological impacts and risk 298 6.1.1 Intentional storage of CO2 in the ocean 279 6.7.2 Physiological effects of CO2 301 6.1.2 Relevant background in physical and chemical 6.7.3 From physiological mechanisms to ecosystems 305 oceanography 281 6.7.4 Biological consequences for water column release scenarios 306 6.2 Approaches to release CO2 into the ocean 282 6.7.5 Biological consequences associated with CO2 6.2.1 Approaches to releasing CO2 that has been captured, lakes 307 compressed, and transported into the ocean 282 6.7.6 Contaminants in CO2 streams 307 6.2.2 CO2 storage by dissolution of carbonate minerals 290 6.7.7 Risk management 307 6.2.3 Other ocean storage approaches 291 6.7.8 Social aspects; public and stakeholder perception 307 6.3 Capacity and fractions retained -
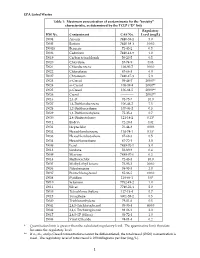
EPA Listed Wastes Table 1: Maximum Concentration of Contaminants For
EPA Listed Wastes Table 1: Maximum concentration of contaminants for the toxicity characteristic, as determined by the TCLP (D list) Regulatory HW No. Contaminant CAS No. Level (mg/L) D004 Arsenic 7440-38-2 5.0 D005 Barium 7440-39-3 100.0 D0018 Benzene 71-43-2 0.5 D006 Cadmium 7440-43-9 1.0 D019 Carbon tetrachloride 56-23-5 0.5 D020 Chlordane 57-74-9 0.03 D021 Chlorobenzene 108-90-7 100.0 D022 Chloroform 67-66-3 6.0 D007 Chromium 7440-47-3 5.0 D023 o-Cresol 95-48-7 200.0** D024 m-Cresol 108-39-4 200.0** D025 p-Cresol 106-44-5 200.0** D026 Cresol ------------ 200.0** D016 2,4-D 94-75-7 10.0 D027 1,4-Dichlorobenzene 106-46-7 7.5 D028 1,2-Dichloroethane 107-06-2 0.5 D029 1,1-Dichloroethylene 75-35-4 0.7 D030 2,4-Dinitrotoluene 121-14-2 0.13* D012 Endrin 72-20-8 0.02 D031 Heptachlor 76-44-8 0.008 D032 Hexachlorobenzene 118-74-1 0.13* D033 Hexachlorobutadiene 87-68-3 0.5 D034 Hexachloroethane 67-72-1 3.0 D008 Lead 7439-92-1 5.0 D013 Lindane 58-89-9 0.4 D009 Mercury 7439-97-6 0.2 D014 Methoxychlor 72-43-5 10.0 D035 Methyl ethyl ketone 78-93-3 200.0 D036 Nitrobenzene 98-95-3 2.0 D037 Pentachlorophenol 87-86-5 100.0 D038 Pyridine 110-86-1 5.0* D010 Selenium 7782-49-2 1.0 D011 Silver 7740-22-4 5.0 D039 Tetrachloroethylene 127-18-4 0.7 D015 Toxaphene 8001-35-2 0.5 D040 Trichloroethylene 79-01-6 0.5 D041 2,4,5-Trichlorophenol 95-95-4 400.0 D042 2,4,6-Trichlorophenol 88-06-2 2.0 D017 2,4,5-TP (Silvex) 93-72-1 1.0 D043 Vinyl Chloride 74-01-4 0.2 * Quantitation limit is greater than the calculated regulatory level. -

Titanium Dioxide Production Final Rule: Mandatory Reporting of Greenhouse Gases
Titanium Dioxide Production Final Rule: Mandatory Reporting of Greenhouse Gases Under the Mandatory Reporting of Greenhouse Gases (GHGs) rule, owners or operators of facilities that contain titanium dioxide production processes (as defined below) must report emissions from titanium dioxide production and all other source categories located at the facility for which methods are defined in the rule. Owners or operators are required to collect emission data; calculate GHG emissions; and follow the specified procedures for quality assurance, missing data, recordkeeping, and reporting. How Is This Source Category Defined? The titanium dioxide production source category consists of any facility that uses the chloride process to produce titanium dioxide. What GHGs Must Be Reported? Each titanium dioxide production facility must report carbon dioxide (CO2) process emissions from each chloride process line. In addition, each facility must report GHG emissions for other source categories for which calculation methods are provided in the rule. For example, facilities must report CO2, nitrous oxide (N2O), and methane (CH4) emissions from each stationary combustion unit on site by following the requirements of 40 CFR part 98, subpart C (General Stationary Fuel Combustion Sources). Please refer to the relevant information sheet for a summary of the rule requirements for calculating and reporting emissions from any other source categories at the facility. How Must GHG Emissions Be Calculated? Reporters must calculate CO2 process emissions using one of two methods, as appropriate: • Installing and operating a continuous emission monitoring system (CEMS) according to the requirements specified in 40 CFR part 98, subpart C. • Calculating the process CO2 emissions for each process line using monthly measurements of the mass and carbon content of calcined petroleum coke. -

Recent Advances in Tio2-Based Photocatalysts for Reduction of CO2 to Fuels
nanomaterials Review Recent Advances in TiO2-Based Photocatalysts for Reduction of CO2 to Fuels 1,2, 3, 4 5 Thang Phan Nguyen y, Dang Le Tri Nguyen y , Van-Huy Nguyen , Thu-Ha Le , Dai-Viet N. Vo 6 , Quang Thang Trinh 7 , Sa-Rang Bae 8, Sang Youn Chae 9,* , Soo Young Kim 8,* and Quyet Van Le 3,* 1 Laboratory of Advanced Materials Chemistry, Advanced Institute of Materials Science, Ton Duc Thang University, Ho Chi Minh City 700000, Vietnam; [email protected] 2 Faculty of Applied Sciences, Ton Duc Thang University, Ho Chi Minh City 700000, Vietnam 3 Institute of Research and Development, Duy Tan University, Da Nang 550000, Vietnam; [email protected] 4 Key Laboratory of Advanced Materials for Energy and Environmental Applications, Lac Hong University, Bien Hoa 810000, Vietnam; [email protected] 5 Faculty of Materials Technology, Ho Chi Minh City University of Technology (HCMUT), Vietnam National University–Ho Chi Minh City (VNU–HCM), 268 Ly Thuong Kiet, District 10, Ho Chi Minh City 700000, Vietnam; [email protected] 6 Center of Excellence for Green Energy and Environmental Nanomaterials (CE@GrEEN), Nguyen Tat Thanh University, 300A Nguyen Tat Thanh, District 4, Ho Chi Minh City 755414, Vietnam; [email protected] 7 Cambridge Centre for Advanced Research and Education in Singapore (CARES), Campus for Research Excellence and Technological Enterprise (CREATE), 1 Create Way, Singapore 138602, Singapore; [email protected] 8 Department of Materials Science and Engineering, Korea University, 145 Anam-ro, Seongbuk-gu, Seoul 02841, Korea; [email protected] 9 Department of Materials Science, Institute for Surface Science and Corrosion, University of Erlangen-Nuremberg, Martensstrasse 7, 91058 Erlangen, Germany * Correspondence: [email protected] (S.Y.C.); [email protected] (S.Y.K.); [email protected] (Q.V.L.); Tel.: +42-01520-2145321 (S.Y.C.); +82-109-3650-910 (S.Y.K.); +84-344-176-848 (Q.V.L.) These authors contributed equally to this work. -

Orofacial Myology Is a Specialized Professional Discipline That Evaluates and Treats a Variety Of
What is Orofacial Myology? Orofacial myology is a specialized professional discipline that evaluates and treats a variety of oral and facial (orofacial) muscles, (myo-) postural and functional disorders and oral habits that may disrupt normal dental development and also create cosmetic problems. The principles involved with the evaluation and treatment of orofacial Myofunctional disorders are based upon dental science tenets; however, orofacial Myofunctional therapy is not dental treatment. Myofunctional therapy can be basically described as correcting an oro-facial muscular unbalance, including correction of the position of the tongue at rest and during swallowing. Specific treatments involve establishing and stabilizing normal rest position of the tongue and lips, eliminating deviate (abnormal) oral habits and correcting swallowing patterns when tongue thrusting is involved. Improvements in appearance are observed during and following therapy. What are Myofunctional disorders and how are they corrected? An oral Myofunctional disorder includes a variety of oral habits, postures and functional activities that may open the normal dental bite or may lead to deformation of the dental arches. • Thumb and finger sucking • an open-mouth posture with lips apart • a forward rest posture of the tongue • Tongue thrusting during speaking and swallowing Above mentioned oral habits characterize Myofunctional disorders. Such disorders can lead to a disruption of normal dental development in both children and adults. The consequence of postural and functional variations involving the lips and tongue are associated with dental malocclusion, cosmetic problems, and deformities in the growth of the dental arches. How Prevalent Are Orofacial Myofunctional Disorders (OMD)? Research examining various populations found 38% have orofacial Myofunctional disorders and, as mentioned above, an incidence of 81% has been found in children exhibiting speech/articulation problems. -

Carbon Dioxide Exposure Effects – Fact Sheet
CARBON DIOXIDE EXPOSURE EFFECTS – FACT SHEET Studies by NIOSH in 1976 dispelled the myth that carbon dioxide is an asphyxiant gas and only causes adverse health effects when it displaces oxygen. Symptoms of overexposure by inhalation include dizziness, headache, nausea, rapid breathing, shortness of breath, deeper breathing, increased heart rate (tachycardia), eye and extremity twitching, cardiac arrhythmia, memory disturbances, lack of concentration, visual and hearing disturbances (including photophobia, blurred vision, transient blindness, hearing loss and ringing in the ears), sweating, restlessness, vomiting, shaking, confusion, flushed skin, panic, parathesis (a sensation of numbness in the extremities), disorientation, convulsions, unconsciousness, coma, and death. CO2 Duration Physiological Impact/Health Effect Concentration 1,000 ppm Less than Impairs judgment, decision-making ability, and thinking skills on a 2½ hrs. short-term basis, even for healthy individuals. 2,500 ppm Less than Many individuals are rendered cognitively marginal or 2½ hrs. dysfunctional. 5,000 ppm with Headache, lethargy, mental slowness, emotional irritation, and 20.9% Oxygen sleep disruption. 6% 1-2 mins. Hearing and visual disturbances 7% (70,000 ppm) 5 mins. death with 20.9% Oxygen 10% to 15% Dizziness, drowsiness, severe muscle twitching, unconsciousness and death within a few minutes. Within 1 Loss of controlled and purposeful activity, unconsciousness, coma, 17% to 30% min. convulsions, and death 30% carbon 30 secs. Unconsciousness, with some subjects having seizures that were dioxide, with 70% characterized as decerebrate (no cerebral functioning). oxygen Even though oxygen is necessary to carry out cell functions, it is not the lack of oxygen that stimulates breathing. Breathing is stimulated by an excess of CO2. -

What Are the Health Effects from Exposure to Carbon Monoxide?
CO Lesson 2 CARBON MONOXIDE: LESSON TWO What are the Health Effects from Exposure to Carbon Monoxide? LESSON SUMMARY Carbon monoxide (CO) is an odorless, tasteless, colorless and nonirritating Grade Level: 9 – 12 gas that is impossible to detect by an exposed person. CO is produced by the Subject(s) Addressed: incomplete combustion of carbon-based fuels, including gas, wood, oil and Science, Biology coal. Exposure to CO is the leading cause of fatal poisonings in the United Class Time: 1 Period States and many other countries. When inhaled, CO is readily absorbed from the lungs into the bloodstream, where it binds tightly to hemoglobin in the Inquiry Category: Guided place of oxygen. CORE UNDERSTANDING/OBJECTIVES By the end of this lesson, students will have a basic understanding of the physiological mechanisms underlying CO toxicity. For specific learning and standards addressed, please see pages 30 and 31. MATERIALS INCORPORATION OF TECHNOLOGY Computer and/or projector with video capabilities INDIAN EDUCATION FOR ALL Fires utilizing carbon-based fuels, such as wood, produce carbon monoxide as a dangerous byproduct when the combustion is incomplete. Fire was important for the survival of early Native American tribes. The traditional teepees were well designed with sophisticated airflow patterns, enabling fires to be contained within the shelter while minimizing carbon monoxide exposure. However, fire was used for purposes other than just heat and cooking. According to the historian Henry Lewis, Native Americans used fire to aid in hunting, crop management, insect collection, warfare and many other activities. Today, fire is used to heat rocks used in sweat lodges. -

Guidelines Proposal for Clinical Recognition of Mouth Breathing Children
original article Guidelines proposal for clinical recognition of mouth breathing children Maria Christina Thomé Pacheco1, Camila Ferreira Casagrande2, Lícia Pacheco Teixeira3, Nathalia Silveira Finck4, Maria Teresa Martins de Araújo5 DOI: http://dx.doi.org/10.1590/2176-9451.20.4.039-044.oar Introduction: Mouth breathing (MB) is an etiological factor for sleep-disordered breathing (SDB) during childhood. The habit of breathing through the mouth may be perpetuated even after airway clearance. Both habit and obstruction may cause facial muscle imbalance and craniofacial changes. Objective: The aim of this paper is to propose and test guidelines for clinical recognition of MB and some predisposing factors for SDB in children. Methods: Semi-structured interviews were conducted with 110 orthodontists regarding their procedures for clinical evaluation of MB and their knowledge about SDB during childhood. Thereafter, based on their answers, guidelines were developed and tested in 687 children aged between 6 and 12 years old and attending elementary schools. Results: There was no standardization for clinical recognition of MB among orthodontists. The most common procedures performed were inefficient to rec- ognize differences between MB by habit or obstruction. Conclusions: The guidelines proposed herein facilitate clinical recognition of MB, help clinicians to differentiate between habit and obstruction, suggest the most appropriate treatment for each case, and avoid maintenance of mouth breathing patterns during adulthood. Keywords: Mouth breathing. Airway obstruction. Craniofacial abnormalities. Introdução: a respiração bucal (RB) é um fator etiológico para os distúrbios respiratórios do sono (DRS) na infância. O hábito de respirar pela boca pode ser perpetuado mesmo depois da desobstrução das vias aéreas. -

Water-Carbon Dioxide Solid Phase Equilibria at Pressures Above 4 Gpa E
www.nature.com/scientificreports OPEN Water-carbon dioxide solid phase equilibria at pressures above 4 GPa E. H. Abramson , O. Bollengier & J. M. Brown A solid phase in the mixed water-carbon dioxide system, previously identified as carbonic acid, was Received: 6 January 2017 observed in the high-pressure diamond-anvil cell. The pressure-temperature paths of both its melting Accepted: 16 March 2017 and peritectic curves were measured, beginning at 4.4 GPa and 165 °C (where it exists in a quadruple Published: xx xx xxxx equilibrium, together with an aqueous fluid and the ices H2O(VII) and CO2(I)) and proceeding to higher pressures and temperatures. Single-crystal X-ray diffraction revealed a triclinic crystal with unit cell parameters (at 6.5 GPa and 20 °C) of a = 5.88 Å, b = 6.59 Å, c = 6.99 Å, α = 88.7°, β = 79.7°, and γ = 67.7°. Raman spectra exhibit a major line at ~1080 cm−1 and lattice modes below 300 cm−1. The binary water-carbon dioxide system is of broad interest, ubiquitous in Earth and planetary sciences, in indus- trial chemistry and in life sciences, and has been the subject of extensive experimental and theoretical attention. Mixed solid phases known from experiments include the sI clathrate hydrate which is stable at temperatures below 21 °C and pressures below 0.7 GPa, and a filled ice observed at pressures up to ~1 GPa, above which it 1–3 decomposes into H2O and CO2 ices . The 1:1 adduct, carbonic acid (H2CO3), has been described in the gas phase, rare-gas -matrix studies and aqueous solution (see, for example, references in Mitterdorfer et al.4 and in Reisenauer et al.5).