A Coclinical Radiogenomic Validation Study
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Promotion of Periostin Expression Contributes to the Migration of Schwann Cells Eva Sonnenberg-Riethmacher1,2,*, Michaela Miehe2,3,* and Dieter Riethmacher1,2,‡
© 2015. Published by The Company of Biologists Ltd | Journal of Cell Science (2015) 128, 3345-3355 doi:10.1242/jcs.174177 RESEARCH ARTICLE Promotion of periostin expression contributes to the migration of Schwann cells Eva Sonnenberg-Riethmacher1,2,*, Michaela Miehe2,3,* and Dieter Riethmacher1,2,‡ ABSTRACT Michailov et al., 2004; Riethmacher et al., 1997; Taveggia et al., Neuregulin ligands and their ErbB receptors are important for the 2005). Those experiments revealed that, later in development, development of Schwann cells, the glial cells of the peripheral mutant embryos exhibit a total loss of Schwann cells along their nervous system (PNS). ErbB3 deficiency is characterized by a peripheral axons, whereas pre-migratory Schwann cells are present complete loss of Schwann cells along axons of the peripheral nerves, near to the dorsal root ganglia but fail to migrate along the axons. impaired fasciculation and neuronal cell death. We performed The impaired glial cell migration in these mutants indicates a direct comparative gene expression analysis of dorsal root ganglia (DRG) function in migration. This interpretation is supported by the finding – explant cultures from ErbB3-deficient and wild-type mice in order to that mutations in the neuregulin ErbB signalling system lead to identify genes that are involved in Schwann cell development and changes in neural crest migration in mice (Britsch et al., 1998) and migration. The extracellular matrix (ECM) gene periostin was found to in directed Schwann cell migration in zebrafish (Lyons et al., 2005). exhibit the most prominent down regulation in ErbB3-deficient DRG. However, an earlier function in fate specification and/or Expression analysis revealed that the periostin-expressing cell proliferation that could in turn be a prerequisite for migration population in the PNS corresponds to Schwann cell precursors and cannot be completely ruled out. -
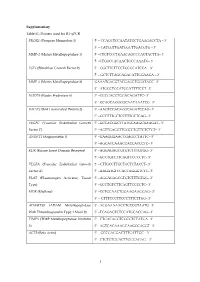
1 Supplementary Table S1. Primers Used for RT-Qpcr PROX1
Supplementary Table S1. Primers used for RT-qPCR PROX1 (Prospero Homeobox 1) 5’ – CCAGCTCCAATATGCTGAAGACCTA – 3’ 5’ – CATCGTTGATGGCTTGACGTG – 3‘ MMP-1 (Matrix Metallopeptidase 1) 5' –CTGTCCCTGAACAGCCCAGTACTTA– 3' 5' –CTGGCCACAACTGCCAAATG– 3' FGF2 (Fibroblast Growth Factor 2) 5′ - GGCTTCTTCCTGCGCATCCA – 3′ 5′ – GCTCTTAGCAGACATTGGAAGA – 3′ MMP-3 (Matrix Metallopeptidase 3) GAAATGAGGTACGAGCTGGATACC– 3’ 5’ –ATGGCTGCATCGATTTTCCT– 3’ NUDT6 (Nudix Hydrolase 6) 5’ –GGCGAGCTGGACAGATTC– 3’ 5’ –GCAGCAGGGGCAATAAATCG– 3’ BAIAP2 (BAI1 Associated Protein 2) 5’ –AAGTCCACAGGCAGATCCAG– 3’ 5’ –GCCTTTGCTCCTTTGCTCAG– 3’ VEGFC (Vascular Endothelial Growth 5’ –GCCACGGCTTATGCAAGCAAAGAT– 3’ Factor C) 5’ –AGTTGAGGTTGGCCTGTTCTCTGT– 3’ ANGPT1 (Angiopoietin 1) 5’ –GAAGGGAACCGAGCCTATTC– 3’ 5’ –AGCATCAAACCACCATCCTC– 3’ KDR (Kinase Insert Domain Receptor) 5’ –AGGAGAGCGTGTCTTTGTGG– 3’ 5’ –GCCTGTCTTCAGTTCCCCTC– 3’ VEGFA (Vascular Endothelial Growth 5’ –CTTGCCTTGCTGCTCTACCT– 3’ Factor A) 5’ –AAGATGTCCACCAGGGTCTC– 3’ PLAT (Plasminogen Activator, Tissue 5’ –AGGAGAGCGTGTCTTTGTGG– 3’ Type) 5’ –GCCTGTCTTCAGTTCCCCTC– 3’ MDK (Midkine) 5’ –CCTGCAACTGGAAGAAGGAG– 3’ 5’ -- CTTTCCCTTCCCTTTCTTGG– 3’ ADAMTS9 (ADAM Metallopeptidase 5’ –ACGAAAAACCTGCCGTAATG– 3’ With Thrombospondin Type 1 Motif 9) 5’ –TCAGAGTCTCCATGCACCAG– 3’ TIMP3 (TIMP Metallopeptidase Inhibitor 5’ –CTGACAGGTCGCGTCTATGA– 3’ 3) 5’ –AGTCACAAAGCAAGGCAGGT– 3’ ACTB (Beta Actin) 5’ – GCCGAGGACTTTGATTGC – 3’ 5’– CTGTGTGGACTTGGGAGAG – 3’ 1 Figure S1. Efficient silencing of PROX1 in CGTH-W-1 and FTC-133 cells. Western blotting analysis shows a decrease in PROX1 protein level by targeting with siRNAs purchased from Santa Cruz (SC) and Sigma-Aldrich (SA) in both CGTH-W-1 and FTC-133 cell line. Beta-actin was used as a loading control of protein lysates. Figure S2. The tube formation assay. The silencing of PROX1 in CGTH-W-1 and FTC-133 cells enhances the angiogenesis in vitro of endothelial cells. HUVECs were cultured in 96-well plates coated with a semi-solid Matrigel. -

Periostin Modulates Myofibroblast Differentiation During Full-Thickness Cutaneous Wound Repair
Research Article 121 Periostin modulates myofibroblast differentiation during full-thickness cutaneous wound repair Christopher G. Elliott1, Jian Wang2, Xiaolei Guo3, Shi-wen Xu4, Mark Eastwood5, Jianjun Guan3, Andrew Leask6, Simon J. Conway2 and Douglas W. Hamilton1,6,* 1Department of Anatomy and Cell Biology, Schulich School of Medicine and Dentistry, The University of Western Ontario, 1151 Richmond St, London, Ontario, Canada, N6A 5C1 2Riley Heart Research Center, Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, 1044 West Walnut, Indianapolis, IN 46202, USA 3Department of Materials Science and Engineering, The Ohio State University, 2041 College Road, Columbus, OH 43210, USA 4Centre for Rheumatology, Royal Free and University College Medical School, London, NW3 2PF, UK 5School of Life Sciences, University of Westminster, London, W1B 2UW, UK 6Division of Oral Biology, Schulich School of Medicine and Dentistry, The University of Western Ontario, 1151 Richmond St, London, Ontario, Canada, N6A 5C1 *Author for correspondence ([email protected]) Accepted 22 July 2011 Journal of Cell Science 125, 121–132 ß 2012. Published by The Company of Biologists Ltd doi: 10.1242/jcs.087841 Summary The matricellular protein periostin is expressed in the skin. Although periostin has been hypothesized to contribute to dermal homeostasis and repair, this has not been directly tested. To assess the contribution of periostin to dermal healing, 6 mm full-thickness excisional wounds were created in the skin of periostin-knockout and wild-type, sex-matched control mice. In wild-type mice, periostin was potently induced 5–7 days after wounding. In the absence of periostin, day 7 wounds showed a significant reduction in myofibroblasts, as visualized by expression of a-smooth muscle actin (a-SMA) within the granulation tissue. -

Gene Alterations Identified by Expression Profiling in Tumor-Associated Endothelial Cells from Invasive Ovarian Carcinoma
Research Article Gene Alterations Identified by Expression Profiling in Tumor-Associated Endothelial Cells from Invasive Ovarian Carcinoma Chunhua Lu,1 Tomas Bonome,3 Yang Li,1 Aparna A. Kamat,1 Liz Y. Han,1 Rosemarie Schmandt,1 Robert L. Coleman,1 David M. Gershenson,1 Robert B. Jaffe,4 MichaelJ. Birrer, 3 and AnilK. Sood 1,2 Departments of 1Gynecologic Oncology and 2Cancer Biology, University of Texas M. D. Anderson Cancer Center, Houston, Texas; 3Cell and Cancer Biology Branch, National Cancer Institute, Bethesda, Maryland; and 4Center for Reproductive Sciences, University of California, San Francisco, San Francisco, California Abstract the promise of such approaches. However, the full spectrum of Therapeutic strategies based on antiangiogenic approaches differences in the tumor vasculature compared with its normal are beginning to show great promise in clinical studies. counterpart is not known. Identification of additional targets on However, full realization of these approaches requires tumor endothelium may allow opportunities for developing new identification of key differences in gene expression between therapeutic approaches to inhibit angiogenesis in a tumor-specific endothelial cells from tumors versus their normal counter- manner. parts. Here, we examined gene expression differences in Higher levels of proangiogenic cytokines and angiogenesis are purified endothelial cells from 10invasive epithelial ovarian associated with an increased risk of metastasis and poor prognosis cancers and 5 normal ovaries using Affymetrix U133 Plus in ovarian cancer (5, 6). To date, a small number of breast, colon, 2.0microarrays. More than 400differentially expressed genes and brain cancers have been analyzed for gene expression changes were identified in tumor-associated endothelial cells. -

Deletion of Periostin Reduces Muscular Dystrophy and Fibrosis in Mice By
Deletion of periostin reduces muscular dystrophy and fibrosis in mice by modulating the transforming growth factor-β pathway Angela Lortsa,1, Jennifer A. Schwanekampa,1, Troy A. Baudinob, Elizabeth M. McNallyc, and Jeffery D. Molkentina,d,2 aDepartment of Pediatrics, Cincinnati Children’s Hospital, University of Cincinnati, Cincinnati, OH 45229; bDepartment of Medicine, Texas A&M Health Science Center College of Medicine, Temple, TX 76504; cDepartment of Medicine, University of Chicago, Chicago, IL 60637; and dHoward Hughes Medical Institute, Cincinnati Children’s Hospital, Cincinnati, OH 45229 Edited by Kevin P. Campbell, University of Iowa Carver College of Medicine, Iowa City, IA, and approved May 18, 2012 (received for review March 20, 2012) The muscular dystrophies are broadly classified as muscle wasting induced and secreted into the ECM after acute injury, as well as diseases with myofiber dropout due to cellular necrosis, inflam- in dystrophic skeletal muscle (13). Periostin is expressed exclu- mation, alterations in extracellular matrix composition, and fatty sively by fibroblasts or cells that adopt a fibroblast-like phenotype cell replacement. These events transpire and progress despite after an injurious event (14). In the heart, we and others have ongoing myofiber regeneration from endogenous satellite cells. shown that periostin is strongly induced after myocardial in- The degeneration/regeneration response to muscle injury/disease farction and left ventricular pressure overload, where it plays is modulated by the proinflammatory cytokine transforming a role in ECM remodeling and healing (15, 16). growth factor-β (TGF-β), which can also profoundly influence ex- In the present study, we found that mice lacking periostin tracellular matrix composition through increased secretion of pro- (Postn) were profoundly protected from MD through mecha- fibrotic proteins, such as the matricellular protein periostin. -

The Inter-Relationship of Periostin, Tgfβ, and BMP in Heart Valve Development and Valvular Heart Diseases
Review TheScientificWorldJOURNAL (2011) 11, 1509–1524 TSW Development & Embryology ISSN 1537-744X; DOI 10.1100/tsw.2011.132 The Inter-Relationship of Periostin, TGFβ, and BMP in Heart Valve Development and Valvular Heart Diseases Simon J. Conway1, Thomas Doetschman2,3, and Mohamad Azhar2,3,* 1Riley Heart Research Center, Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis; 2BIO5 Institute, University of Arizona, Tucson; 3Department of Cellular and Molecular Medicine, University of Arizona, Tucson E-mail: [email protected] Received August 23, 2009; Revised June 28, 2011; Accepted July 4, 2011; Published July 28, 2011 Recent studies have suggested an important role for periostin and transforming growth factor beta (TGFβ) and bone morphogenetic protein (BMP) ligands in heart valve formation and valvular heart diseases. The function of these molecules in cardiovascular development has previously been individually reviewed, but their association has not been thoroughly examined. Here, we summarize the current understanding of the association between periostin and TGFβ and BMP ligands, and discuss the implications of this association in the context of the role of these molecules in heart valve development and valvular homeostasis. Information about hierarchal connections between periostin and TGFβ and BMP ligands in valvulogenesis will increase our understanding of the pathogenesis, progression, and medical treatment of human valve diseases. KEYWORDS: periostin, transforming growth factor beta, TGFβ, bone morphogenetic protein, BMP, heart development, heart valves, Marfan syndrome INTRODUCTION About one-fourth of patients with congenital heart disease, which affects approximately 5% of live births, have structural anomalies of one or more heart valves[1,2]. Isolated bicuspid aortic valve and congenital polyvalvular disease represent major congenital cardiac valve malformations[3,4,5]. -

Periostin Induces Proliferation of Differentiated Cardiomyocytes and Promotes Cardiac Repair
ARTICLES Periostin induces proliferation of differentiated cardiomyocytes and promotes cardiac repair Bernhard Ku¨hn1, Federica del Monte2, Roger J Hajjar2,4, Yuh-Shin Chang3, Djamel Lebeche2,4, Shima Arab1 & Mark T Keating1,4 Adult mammalian hearts respond to injury with scar formation and not with cardiomyocyte proliferation, the cellular basis of regeneration. Although cardiogenic progenitor cells may maintain myocardial turnover, they do not give rise to a robust regenerative response. Here we show that extracellular periostin induced reentry of differentiated mammalian cardiomyocytes into the cell cycle. Periostin stimulated mononucleated cardiomyocytes to go through the full mitotic cell cycle. Periostin activated aV, b1, b3 and b5 integrins located in the cardiomyocyte cell membrane. Activation of phosphatidylinositol-3-OH kinase was required for periostin- http://www.nature.com/naturemedicine induced reentry of cardiomyocytes into the cell cycle and was sufficient for cell-cycle reentry in the absence of periostin. After myocardial infarction, periostin-induced cardiomyocyte cell-cycle reentry and mitosis were associated with improved ventricular remodeling and myocardial function, reduced fibrosis and infarct size, and increased angiogenesis. Thus, periostin and the pathway that it regulates may provide a target for innovative strategies to treat heart failure. Differentiated cardiomyocytes carry the pump function of the heart. hypertrophic cardiomyopathy16. By contrast, liposomal gene delivery Loss of cardiomyocytes, which occurs, for example, after a large of periostin leads to dilative cardiomyopathy without hypertrophy17. myocardial infarction, typically results in heart failure. Observations Here we have investigated whether recombinant periostin, delivered spanning the twentieth century show that some cardiomyocytes in the through the cardiac extracellular matrix, can increase cardiomyocyte adult heart undergo DNA synthesis and mitosis1,2. -

Periostin Is Required for the Maintenance of Muscle Fibers During Muscle Regeneration
International Journal of Molecular Sciences Article Periostin Is Required for the Maintenance of Muscle Fibers during Muscle Regeneration Naoki Ito 1,2,3 , Yuko Miyagoe-Suzuki 2 , Shin’ichi Takeda 2,* and Akira Kudo 3,* 1 Laboratory of Molecular Life Science, Institute of Biomedical Research and Innovation (IBRI), Foundation for Biomedical Research and Innovation at Kobe (FBRI), Kobe 650-0047, Japan; [email protected] 2 National Center of Neurology and Psychiatry, Department of Molecular Therapy, National Institute of Neuroscience, Tokyo 187-8502, Japan; [email protected] 3 Department of Biological Information, Tokyo Institute of Technology, Yokohama 226-8501, Japan * Correspondence: [email protected] (S.T.); [email protected] (A.K.) Abstract: Skeletal muscle regeneration is a well-organized process that requires remodeling of the extracellular matrix (ECM). In this study, we revealed the protective role of periostin, a matricellular protein that binds to several ECM proteins during muscle regeneration. In intact muscle, periostin was localized at the neuromuscular junction, muscle spindle, and myotendinous junction, which are connection sites between muscle fibers and nerves or tendons. During muscle regeneration, periostin exhibited robustly increased expression and localization at the interstitial space. Periostin-null mice showed decreased muscle weight due to the loss of muscle fibers during repeated muscle regeneration. Cultured muscle progenitor cells from periostin-null mice showed no deficiencies in their proliferation, differentiation, and the expression of Pax7, MyoD, and myogenin, suggesting that the loss of muscle Citation: Ito, N.; Miyagoe-Suzuki, Y.; fibers in periostin-null mice was not due to the impaired function of muscle stem/progenitor cells. -

Periostin Promotes Invasion and Anchorage-Independent Growth in the Metastatic Process of Head and Neck Cancer
Research Article Periostin Promotes Invasion and Anchorage-Independent Growth in the Metastatic Process of Head and Neck Cancer Yasusei Kudo,1 Ikuko Ogawa,2 Shojiro Kitajima,1 Masae Kitagawa,1 Hidehiko Kawai,3 Patrick M. Gaffney,4 Mutsumi Miyauchi,1 and Takashi Takata1,2 1Department of Oral Maxillofacial Pathobiology, Division of Frontier Medical Science, Graduate School of Biomedical Sciences, Hiroshima University, 2Center of Oral Clinical Examination, Hiroshima University Hospital, 3Department of Regulatory Radiobiology, Research Institute for Radiation Biology and Medicine, Hiroshima University, Hiroshima, Japan; and 4Division of Hematology, Oncology and Transplantation, Institute of Human Genetics, Department of Medicine, University of Minnesota Medical School, Minneapolis, Minnesota Abstract tion, prevention, and treatment. Attempts to identify the genes Head and neck squamous cell carcinoma (HNSCC) is one of involved in the metastasis are pivotal for the early prediction of the most common types of human cancer. Typically, HNSCC HNSCC behavior. However, the identity and time of onset of the cells show persistent invasion that frequently leads to local alterations that endow cancer cells with these metastatic functions recurrence and distant lymphatic metastasis. However, mo- are largely unknown. The process of metastasis consists of lecular mechanisms associated with the invasion and metas- sequential and selective steps including proliferation, induction tasis of HNSCC remain poorly understood. Here, we identified of angiogenesis, detachment, motility, invasion into circulation, aggregation and survival in the circulation, cell arrest in distant periostin as an invasion-promoting factor in HNSCC by comparing the gene expression profiles between parent capillary beds, and extravasation into organ parenchyma (3). The HNSCC cells and a highly invasive clone. -

Nfkb-Induced Periostin Activates Integrin-B3 Signaling to Promote Renal Injury in GN
BASIC RESEARCH www.jasn.org NFkB-Induced Periostin Activates Integrin-b3 Signaling to Promote Renal Injury in GN † †‡ Niki Prakoura,* Panagiotis Kavvadas,* Raphaёl Kormann,* Jean-Claude Dussaule,* † † Christos E. Chadjichristos,* and Christos Chatziantoniou* *Institut National de la Santé Et de la Recherche Médicale, Unité Mixte de Recherche Scientifique 1155, Tenon Hospital, Paris, France; †Sorbonne Université, Université Pierre-et-Marie-Curie Paris 6, Paris, France; and ‡Department of Physiology, Saint-Antoine Hospital, Assistance Publique-Hôpitaux de Paris, Paris, France ABSTRACT De novo expression in the kidney of periostin, a protein involved in odontogenesis and osteogenesis, has been suggested as a biomarker of renal disease. In this study, we investigated the mechanism(s) of induction and the role of periostin in renal disease. Using a combination of bioinformatics, reporter assay, and chromatin immunoprecipitation analyses, we found that NFkB and other proinflammatory transcription factors induce periostin expression in vitro and that binding of these factors on the periostin promoter is enriched in glo- meruli during experimental GN. Mice lacking expression of periostin displayed preserved renal function and structure during GN. Furthermore, delayed administration of periostin antisense oligonucleotides in wild-type animals with GN reversed already established proteinuria, diminished tissue inflammation, and improved renal structure. Lack of periostin expression also blunted the de novo renal expression of integrin-b3and phosphorylation of focal adhesion kinase and AKT, known mediators of integrin-b3 signaling that affect cell motility and survival, observed during GN in wild-type animals. In vitro, recombinant periostin increased the expression of integrin-b3 and the concomitant phosphorylation of focal adhesion kinase and AKT in podo- cytes. -

Periostin and Epithelial–Mesenchymal Transition Score As
Published OnlineFirst March 3, 2020; DOI: 10.1158/1078-0432.CCR-19-2297 CLINICAL CANCER RESEARCH | PRECISION MEDICINE AND IMAGING Periostin and Epithelial–Mesenchymal Transition Score as Novel Prognostic Markers for Leiomyosarcoma, Myxofibrosarcoma, and Undifferentiated Pleomorphic Sarcoma Maria Assunta Piano1, Antonella Brunello2, Rocco Cappellesso3, Paola Del Bianco4, Adriana Mattiolo1, Chiara Fritegotto1, Barbara Montini1, Carolina Zamuner5, Paolo Del Fiore6, Marco Rastrelli6, Antonio Sommariva6, Gian Luca De Salvo4, Maria Cristina Montesco5, Carlo Riccardo Rossi6,7, Vittorina Zagonel2, and Maria Luisa Calabro1 ABSTRACT ◥ Purpose: Interpatient clinical variability in soft-tissue sarcomas Results: High expression of periostin, a mesenchymal matri- (STS) highlights the need for novel prognostic markers supporting cellular protein, in sarcoma tissues (P ¼ 0.0024), its high patient risk stratification. As sarcomas might exhibit a more mes- stromal accumulation in leiomyosarcomas (P ¼ 0.0075), and enchymal or a more epithelial state, we focused on epithelial– increased circulation (>20 ng/mL, P ¼ 0.0008) were associated mesenchymal and mesenchymal–epithelial transitions (EMT/ with reduced OS. High periostin expression [HR 2.9; 95% MET) for prognostic clues, and selected three histotypes with confidence interval (CI), 1.3–6.9; P ¼ 0.0134] and circulation variable aggressiveness. (HR 2.6; 95% CI, 1.3–5.1; P ¼ 0.0086), and a mesenchymal Experimental Design: The expression of EMT/MET-related EMT score (mesenchymal vs. transitioning; HR, 5.2; 95% CI, factors was measured by qRT-PCR in 55 tumor samples from 2.1–13.0, P ¼ 0.0005) were associated with increased risk in patients with leiomyosarcoma, myxofibrosarcoma, or undifferen- multivariable models. An intrinsic or induced mesenchymal tiated pleomorphic sarcoma. -

Periostin (POSTN) Regulates Tumor Resistance to Antiangiogenic Therapy in Glioma Models Soon Young Park1, Yuji Piao1, Kang Jin Jeong2, Jianwen Dong1, and John F
Published OnlineFirst June 15, 2016; DOI: 10.1158/1535-7163.MCT-15-0427 Cancer Biology and Signal Transduction Molecular Cancer Therapeutics Periostin (POSTN) Regulates Tumor Resistance to Antiangiogenic Therapy in Glioma Models Soon Young Park1, Yuji Piao1, Kang Jin Jeong2, Jianwen Dong1, and John F. de Groot1 Abstract Periostin (POSTN) interacts with multiple integrins to volume. Resistance to anti-VEGF-A therapy regulated by coordinate a variety of cellular processes, including epitheli- POSTN was associated with increased expression of TGFb1 al-to-mesenchymal transition (EMT) and cell migration. In and hypoxia-inducible factor-1a (HIF1a)inGSCs.Atthe our previous study, anti-VEGF-A therapy was associated with molecular level, POSTN regulated invasion and expression of resistance and EMT. This study sought to determine the role of EMT (caveolin-1) and angiogenesis-related genes (HIF1a and POSTN in the resistance of glioma stem cells (GSC) to anti- VEGF-A) through activation of STAT3. Moreover, recombinant angiogenic therapy. In mouse xenograft models of human POSTN increased GSC invasion. Collectively, our findings glioma, POSTN expression was associated with acquired resis- suggest that POSTN plays an important role in glioma inva- tance to anti-VEGF-A therapy and had a synergistic effect with sion and resistance to antiangiogenic therapy. Mol Cancer Ther; bevacizumab in prolonging survival and decreasing tumor 15(9); 2187–97. Ó2016 AACR. Introduction vascularization mechanisms may contribute to resistance of glio- blastoma to antiangiogenic therapies (5). Tumor angiogenesis is regulated by angiogenic factors, includ- Periostin (POSTN; osteoblast-specific factor 2) is a 90-kDa ing VEGF, platelet-derived growth factor, and hypoxia-inducible extracellular matrix (ECM) protein containing an amino-terminal factor-1a (HIF1a; ref.