Role of Periostin and Interleukin-4 in Recurrence of Pterygia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Promotion of Periostin Expression Contributes to the Migration of Schwann Cells Eva Sonnenberg-Riethmacher1,2,*, Michaela Miehe2,3,* and Dieter Riethmacher1,2,‡
© 2015. Published by The Company of Biologists Ltd | Journal of Cell Science (2015) 128, 3345-3355 doi:10.1242/jcs.174177 RESEARCH ARTICLE Promotion of periostin expression contributes to the migration of Schwann cells Eva Sonnenberg-Riethmacher1,2,*, Michaela Miehe2,3,* and Dieter Riethmacher1,2,‡ ABSTRACT Michailov et al., 2004; Riethmacher et al., 1997; Taveggia et al., Neuregulin ligands and their ErbB receptors are important for the 2005). Those experiments revealed that, later in development, development of Schwann cells, the glial cells of the peripheral mutant embryos exhibit a total loss of Schwann cells along their nervous system (PNS). ErbB3 deficiency is characterized by a peripheral axons, whereas pre-migratory Schwann cells are present complete loss of Schwann cells along axons of the peripheral nerves, near to the dorsal root ganglia but fail to migrate along the axons. impaired fasciculation and neuronal cell death. We performed The impaired glial cell migration in these mutants indicates a direct comparative gene expression analysis of dorsal root ganglia (DRG) function in migration. This interpretation is supported by the finding – explant cultures from ErbB3-deficient and wild-type mice in order to that mutations in the neuregulin ErbB signalling system lead to identify genes that are involved in Schwann cell development and changes in neural crest migration in mice (Britsch et al., 1998) and migration. The extracellular matrix (ECM) gene periostin was found to in directed Schwann cell migration in zebrafish (Lyons et al., 2005). exhibit the most prominent down regulation in ErbB3-deficient DRG. However, an earlier function in fate specification and/or Expression analysis revealed that the periostin-expressing cell proliferation that could in turn be a prerequisite for migration population in the PNS corresponds to Schwann cell precursors and cannot be completely ruled out. -
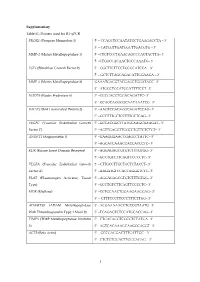
1 Supplementary Table S1. Primers Used for RT-Qpcr PROX1
Supplementary Table S1. Primers used for RT-qPCR PROX1 (Prospero Homeobox 1) 5’ – CCAGCTCCAATATGCTGAAGACCTA – 3’ 5’ – CATCGTTGATGGCTTGACGTG – 3‘ MMP-1 (Matrix Metallopeptidase 1) 5' –CTGTCCCTGAACAGCCCAGTACTTA– 3' 5' –CTGGCCACAACTGCCAAATG– 3' FGF2 (Fibroblast Growth Factor 2) 5′ - GGCTTCTTCCTGCGCATCCA – 3′ 5′ – GCTCTTAGCAGACATTGGAAGA – 3′ MMP-3 (Matrix Metallopeptidase 3) GAAATGAGGTACGAGCTGGATACC– 3’ 5’ –ATGGCTGCATCGATTTTCCT– 3’ NUDT6 (Nudix Hydrolase 6) 5’ –GGCGAGCTGGACAGATTC– 3’ 5’ –GCAGCAGGGGCAATAAATCG– 3’ BAIAP2 (BAI1 Associated Protein 2) 5’ –AAGTCCACAGGCAGATCCAG– 3’ 5’ –GCCTTTGCTCCTTTGCTCAG– 3’ VEGFC (Vascular Endothelial Growth 5’ –GCCACGGCTTATGCAAGCAAAGAT– 3’ Factor C) 5’ –AGTTGAGGTTGGCCTGTTCTCTGT– 3’ ANGPT1 (Angiopoietin 1) 5’ –GAAGGGAACCGAGCCTATTC– 3’ 5’ –AGCATCAAACCACCATCCTC– 3’ KDR (Kinase Insert Domain Receptor) 5’ –AGGAGAGCGTGTCTTTGTGG– 3’ 5’ –GCCTGTCTTCAGTTCCCCTC– 3’ VEGFA (Vascular Endothelial Growth 5’ –CTTGCCTTGCTGCTCTACCT– 3’ Factor A) 5’ –AAGATGTCCACCAGGGTCTC– 3’ PLAT (Plasminogen Activator, Tissue 5’ –AGGAGAGCGTGTCTTTGTGG– 3’ Type) 5’ –GCCTGTCTTCAGTTCCCCTC– 3’ MDK (Midkine) 5’ –CCTGCAACTGGAAGAAGGAG– 3’ 5’ -- CTTTCCCTTCCCTTTCTTGG– 3’ ADAMTS9 (ADAM Metallopeptidase 5’ –ACGAAAAACCTGCCGTAATG– 3’ With Thrombospondin Type 1 Motif 9) 5’ –TCAGAGTCTCCATGCACCAG– 3’ TIMP3 (TIMP Metallopeptidase Inhibitor 5’ –CTGACAGGTCGCGTCTATGA– 3’ 3) 5’ –AGTCACAAAGCAAGGCAGGT– 3’ ACTB (Beta Actin) 5’ – GCCGAGGACTTTGATTGC – 3’ 5’– CTGTGTGGACTTGGGAGAG – 3’ 1 Figure S1. Efficient silencing of PROX1 in CGTH-W-1 and FTC-133 cells. Western blotting analysis shows a decrease in PROX1 protein level by targeting with siRNAs purchased from Santa Cruz (SC) and Sigma-Aldrich (SA) in both CGTH-W-1 and FTC-133 cell line. Beta-actin was used as a loading control of protein lysates. Figure S2. The tube formation assay. The silencing of PROX1 in CGTH-W-1 and FTC-133 cells enhances the angiogenesis in vitro of endothelial cells. HUVECs were cultured in 96-well plates coated with a semi-solid Matrigel. -

Genomic Aberrations Associated with Erlotinib Resistance in Non-Small Cell Lung Cancer Cells
ANTICANCER RESEARCH 33: 5223-5234 (2013) Genomic Aberrations Associated with Erlotinib Resistance in Non-small Cell Lung Cancer Cells MASAKUNI SERIZAWA1, TOSHIAKI TAKAHASHI2, NOBUYUKI YAMAMOTO2,3 and YASUHIRO KOH1 1Drug Discovery and Development Division, Shizuoka Cancer Center Research Institute, Sunto-gun, Shizuoka, Japan; 2Division of Thoracic Oncology, Shizuoka Cancer Center Hospital, Sunto-gun, Shizuoka, Japan; 3Third Department of Internal Medicine, Wakayama Medical University, Kimiidera, Wakayama, Japan Abstract. Background/Aim: Mechanisms of resistance to mutations develop resistance, usually within one year of epidermal growth factor receptor (EGFR)-tyrosine kinase treatment. Therefore, there is an urgent need to elucidate the inhibitors (TKIs) in non-small cell lung cancer (NSCLC) underlying mechanisms of resistance in such tumors to are not fully-understood. In this study we aimed to overcome this obstacle (11-14, 17, 24). Recent studies elucidate remaining unknown mechanisms using erlotinib- suggest that mechanisms of acquired resistance to EGFR- resistant NSCLC cells. Materials and Methods: We TKIs can be categorized into three groups: occurrence of performed array comparative genomic hybridization genetic alterations, activation of downstream pathways via (aCGH) to identify genomic aberrations associated with bypass signaling, and phenotypic transformation (15, 16, 21); EGFR-TKI resistance in erlotinib-resistant PC-9ER cells. therapeutic strategies to overcome these resistance Real-time polymerase chain reaction (PCR) and mechanisms are under development. However, although the immunoblot analyses were performed to confirm the results causes of acquired resistance to EGFR-TKIs have been of aCGH. Results: Among the five regions with copy investigated, in more than 30% of patients with acquired number gain detected in PC-9ER cells, we focused on resistance to EGFR-TKI treatment, the mechanisms remain 22q11.2-q12.1 including v-crk avian sarcoma virus CT10 unknown (15). -

AP1B1 Rabbit Polyclonal Antibody – TA323221 | Origene
OriGene Technologies, Inc. 9620 Medical Center Drive, Ste 200 Rockville, MD 20850, US Phone: +1-888-267-4436 [email protected] EU: [email protected] CN: [email protected] Product datasheet for TA323221 AP1B1 Rabbit Polyclonal Antibody Product data: Product Type: Primary Antibodies Applications: IHC Recommended Dilution: ELISA: 1:1000-5000, IHC: 1:25-100 Reactivity: Human, Mouse, Rat Host: Rabbit Isotype: IgG Clonality: Polyclonal Immunogen: Synthetic peptide corresponding to a region derived from 18-30 amino acids of Human Adapter-related protein complex 1 subunit beta-1 Formulation: PBS pH7.3, 0.05% NaN3, 50% glycerol Concentration: lot specific Purification: Antigen affinity purification Conjugation: Unconjugated Storage: Store at -20°C as received. Stability: Stable for 12 months from date of receipt. Gene Name: adaptor related protein complex 1 beta 1 subunit Database Link: NP_001118 Entrez Gene 11764 MouseEntrez Gene 29663 RatEntrez Gene 162 Human Q10567 Background: Adaptor protein complex 1 is found at the cytoplasmic face of coated vesicles located at the Golgi complex; where it mediates both the recruitment of clathrin to the membrane and the recognition of sorting signals within the cytosolic tails of transmembrane receptors. This complex is a heterotetramer composed of two large; one medium; and one small adaptin subunit. The protein encoded by this gene serves as one of the large subunits of this complex and is a member of the adaptin protein family. This gene is a candidate meningioma gene. Alternative splicing results in multiple transcript variants. Synonyms: ADTB1; AP105A; BAM22; CLAPB2 Protein Pathways: Lysosome This product is to be used for laboratory only. -

Periostin Modulates Myofibroblast Differentiation During Full-Thickness Cutaneous Wound Repair
Research Article 121 Periostin modulates myofibroblast differentiation during full-thickness cutaneous wound repair Christopher G. Elliott1, Jian Wang2, Xiaolei Guo3, Shi-wen Xu4, Mark Eastwood5, Jianjun Guan3, Andrew Leask6, Simon J. Conway2 and Douglas W. Hamilton1,6,* 1Department of Anatomy and Cell Biology, Schulich School of Medicine and Dentistry, The University of Western Ontario, 1151 Richmond St, London, Ontario, Canada, N6A 5C1 2Riley Heart Research Center, Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, 1044 West Walnut, Indianapolis, IN 46202, USA 3Department of Materials Science and Engineering, The Ohio State University, 2041 College Road, Columbus, OH 43210, USA 4Centre for Rheumatology, Royal Free and University College Medical School, London, NW3 2PF, UK 5School of Life Sciences, University of Westminster, London, W1B 2UW, UK 6Division of Oral Biology, Schulich School of Medicine and Dentistry, The University of Western Ontario, 1151 Richmond St, London, Ontario, Canada, N6A 5C1 *Author for correspondence ([email protected]) Accepted 22 July 2011 Journal of Cell Science 125, 121–132 ß 2012. Published by The Company of Biologists Ltd doi: 10.1242/jcs.087841 Summary The matricellular protein periostin is expressed in the skin. Although periostin has been hypothesized to contribute to dermal homeostasis and repair, this has not been directly tested. To assess the contribution of periostin to dermal healing, 6 mm full-thickness excisional wounds were created in the skin of periostin-knockout and wild-type, sex-matched control mice. In wild-type mice, periostin was potently induced 5–7 days after wounding. In the absence of periostin, day 7 wounds showed a significant reduction in myofibroblasts, as visualized by expression of a-smooth muscle actin (a-SMA) within the granulation tissue. -

Gene Alterations Identified by Expression Profiling in Tumor-Associated Endothelial Cells from Invasive Ovarian Carcinoma
Research Article Gene Alterations Identified by Expression Profiling in Tumor-Associated Endothelial Cells from Invasive Ovarian Carcinoma Chunhua Lu,1 Tomas Bonome,3 Yang Li,1 Aparna A. Kamat,1 Liz Y. Han,1 Rosemarie Schmandt,1 Robert L. Coleman,1 David M. Gershenson,1 Robert B. Jaffe,4 MichaelJ. Birrer, 3 and AnilK. Sood 1,2 Departments of 1Gynecologic Oncology and 2Cancer Biology, University of Texas M. D. Anderson Cancer Center, Houston, Texas; 3Cell and Cancer Biology Branch, National Cancer Institute, Bethesda, Maryland; and 4Center for Reproductive Sciences, University of California, San Francisco, San Francisco, California Abstract the promise of such approaches. However, the full spectrum of Therapeutic strategies based on antiangiogenic approaches differences in the tumor vasculature compared with its normal are beginning to show great promise in clinical studies. counterpart is not known. Identification of additional targets on However, full realization of these approaches requires tumor endothelium may allow opportunities for developing new identification of key differences in gene expression between therapeutic approaches to inhibit angiogenesis in a tumor-specific endothelial cells from tumors versus their normal counter- manner. parts. Here, we examined gene expression differences in Higher levels of proangiogenic cytokines and angiogenesis are purified endothelial cells from 10invasive epithelial ovarian associated with an increased risk of metastasis and poor prognosis cancers and 5 normal ovaries using Affymetrix U133 Plus in ovarian cancer (5, 6). To date, a small number of breast, colon, 2.0microarrays. More than 400differentially expressed genes and brain cancers have been analyzed for gene expression changes were identified in tumor-associated endothelial cells. -

University of California Santa Cruz Sample
UNIVERSITY OF CALIFORNIA SANTA CRUZ SAMPLE-SPECIFIC CANCER PATHWAY ANALYSIS USING PARADIGM A dissertation submitted in partial satisfaction of the requirements for the degree of DOCTOR OF PHILOSOPHY in BIOMOLECULAR ENGINEERING AND BIOINFORMATICS by Stephen C. Benz June 2012 The Dissertation of Stephen C. Benz is approved: Professor David Haussler, Chair Professor Joshua Stuart Professor Nader Pourmand Dean Tyrus Miller Vice Provost and Dean of Graduate Studies Copyright c by Stephen C. Benz 2012 Table of Contents List of Figures v List of Tables xi Abstract xii Dedication xiv Acknowledgments xv 1 Introduction 1 1.1 Identifying Genomic Alterations . 2 1.2 Pathway Analysis . 5 2 Methods to Integrate Cancer Genomics Data 10 2.1 UCSC Cancer Genomics Browser . 11 2.2 BioIntegrator . 16 3 Pathway Analysis Using PARADIGM 20 3.1 Method . 21 3.2 Comparisons . 26 3.2.1 Distinguishing True Networks From Decoys . 27 3.2.2 Tumor versus Normal - Pathways associated with Ovarian Cancer 29 3.2.3 Differentially Regulated Pathways in ER+ve vs ER-ve breast can- cers . 36 3.2.4 Therapy response prediction using pathways (Platinum Free In- terval in Ovarian Cancer) . 38 3.3 Unsupervised Stratification of Cancer Patients by Pathway Activities . 42 4 SuperPathway - A Global Pathway Model for Cancer 51 4.1 SuperPathway in Ovarian Cancer . 55 4.2 SuperPathway in Breast Cancer . 61 iii 4.2.1 Chin-Naderi Cohort . 61 4.2.2 TCGA Breast Cancer . 63 4.3 Cross-Cancer SuperPathway . 67 5 Pathway Analysis of Drug Effects 74 5.1 SuperPathway on Breast Cell Lines . -

A Peripheral Blood Gene Expression Signature to Diagnose Subclinical Acute Rejection
CLINICAL RESEARCH www.jasn.org A Peripheral Blood Gene Expression Signature to Diagnose Subclinical Acute Rejection Weijia Zhang,1 Zhengzi Yi,1 Karen L. Keung,2 Huimin Shang,3 Chengguo Wei,1 Paolo Cravedi,1 Zeguo Sun,1 Caixia Xi,1 Christopher Woytovich,1 Samira Farouk,1 Weiqing Huang,1 Khadija Banu,1 Lorenzo Gallon,4 Ciara N. Magee,5 Nader Najafian,5 Milagros Samaniego,6 Arjang Djamali ,7 Stephen I. Alexander,2 Ivy A. Rosales,8 Rex Neal Smith,8 Jenny Xiang,3 Evelyne Lerut,9 Dirk Kuypers,10,11 Maarten Naesens ,10,11 Philip J. O’Connell,2 Robert Colvin,8 Madhav C. Menon,1 and Barbara Murphy1 Due to the number of contributing authors, the affiliations are listed at the end of this article. ABSTRACT Background In kidney transplant recipients, surveillance biopsies can reveal, despite stable graft function, histologic features of acute rejection and borderline changes that are associated with undesirable graft outcomes. Noninvasive biomarkers of subclinical acute rejection are needed to avoid the risks and costs associated with repeated biopsies. Methods We examined subclinical histologic and functional changes in kidney transplant recipients from the prospective Genomics of Chronic Allograft Rejection (GoCAR) study who underwent surveillance biopsies over 2 years, identifying those with subclinical or borderline acute cellular rejection (ACR) at 3 months (ACR-3) post-transplant. We performed RNA sequencing on whole blood collected from 88 indi- viduals at the time of 3-month surveillance biopsy to identify transcripts associated with ACR-3, developed a novel sequencing-based targeted expression assay, and validated this gene signature in an independent cohort. -

Clathrin Heavy and Light Chain Isoforms Originated by Independent Mechanisms of Gene Duplication During Chordate Evolution
Clathrin heavy and light chain isoforms originated by independent mechanisms of gene duplication during chordate evolution Diane E. Wakeham†‡, Laurent Abi-Rached‡§, Mhairi C. Towler†¶, Jeremy D. Wilbur†ʈ, Peter Parham§, and Frances M. Brodsky†,†† †The G. W. Hooper Foundation and Departments of Biopharmaceutical Sciences, Pharmaceutical Chemistry, and Microbiology and Immunology and ʈBiophysics Program, University of California, San Francisco, CA 94143-0552; and §Department of Structural Biology and Department of Microbiology and Immunology, Stanford University, Stanford, CA 94305 Communicated by J. Michael Bishop, University of California, San Francisco, CA, March 22, 2005 (received for review January 14, 2005) In humans, there are two isoforms each of clathrin heavy chain biogenesis. CHC17 is expressed ubiquitously in vertebrate tissues (CHC17 and CHC22) and light chain (LCa and LCb) subunits, all and a functional orthologue is present in all eukaryotic organ- encoded by separate genes. CHC17 forms the ubiquitous clathrin- isms analyzed. The CHC22 isoform is highly expressed in human coated vesicles that mediate membrane traffic. CHC22 is implicated skeletal muscle, with a low level detected in other tissues. CHC22 in specialized membrane organization in skeletal muscle. CHC17 is is concentrated at neuromuscular and myotendinous junctions, bound and regulated by LCa and LCb, whereas CHC22 does not and its expression increases during myogenesis and muscle functionally interact with either light chain. The imbalanced inter- regeneration (10, 11). Thus, CHC22 appears to have a role actions between clathrin subunit isoforms suggest a distinct evo- distinct from CHC17 in specialized muscle membrane organi- lutionary history for each isoform pair. Phylogenetic and sequence zation. CHC17 and CHC22 have a remarkably high protein sequence identity (85%), despite their evident differences in analysis placed both heavy and light chain gene duplications function. -

Deletion of Periostin Reduces Muscular Dystrophy and Fibrosis in Mice By
Deletion of periostin reduces muscular dystrophy and fibrosis in mice by modulating the transforming growth factor-β pathway Angela Lortsa,1, Jennifer A. Schwanekampa,1, Troy A. Baudinob, Elizabeth M. McNallyc, and Jeffery D. Molkentina,d,2 aDepartment of Pediatrics, Cincinnati Children’s Hospital, University of Cincinnati, Cincinnati, OH 45229; bDepartment of Medicine, Texas A&M Health Science Center College of Medicine, Temple, TX 76504; cDepartment of Medicine, University of Chicago, Chicago, IL 60637; and dHoward Hughes Medical Institute, Cincinnati Children’s Hospital, Cincinnati, OH 45229 Edited by Kevin P. Campbell, University of Iowa Carver College of Medicine, Iowa City, IA, and approved May 18, 2012 (received for review March 20, 2012) The muscular dystrophies are broadly classified as muscle wasting induced and secreted into the ECM after acute injury, as well as diseases with myofiber dropout due to cellular necrosis, inflam- in dystrophic skeletal muscle (13). Periostin is expressed exclu- mation, alterations in extracellular matrix composition, and fatty sively by fibroblasts or cells that adopt a fibroblast-like phenotype cell replacement. These events transpire and progress despite after an injurious event (14). In the heart, we and others have ongoing myofiber regeneration from endogenous satellite cells. shown that periostin is strongly induced after myocardial in- The degeneration/regeneration response to muscle injury/disease farction and left ventricular pressure overload, where it plays is modulated by the proinflammatory cytokine transforming a role in ECM remodeling and healing (15, 16). growth factor-β (TGF-β), which can also profoundly influence ex- In the present study, we found that mice lacking periostin tracellular matrix composition through increased secretion of pro- (Postn) were profoundly protected from MD through mecha- fibrotic proteins, such as the matricellular protein periostin. -

The Inter-Relationship of Periostin, Tgfβ, and BMP in Heart Valve Development and Valvular Heart Diseases
Review TheScientificWorldJOURNAL (2011) 11, 1509–1524 TSW Development & Embryology ISSN 1537-744X; DOI 10.1100/tsw.2011.132 The Inter-Relationship of Periostin, TGFβ, and BMP in Heart Valve Development and Valvular Heart Diseases Simon J. Conway1, Thomas Doetschman2,3, and Mohamad Azhar2,3,* 1Riley Heart Research Center, Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis; 2BIO5 Institute, University of Arizona, Tucson; 3Department of Cellular and Molecular Medicine, University of Arizona, Tucson E-mail: [email protected] Received August 23, 2009; Revised June 28, 2011; Accepted July 4, 2011; Published July 28, 2011 Recent studies have suggested an important role for periostin and transforming growth factor beta (TGFβ) and bone morphogenetic protein (BMP) ligands in heart valve formation and valvular heart diseases. The function of these molecules in cardiovascular development has previously been individually reviewed, but their association has not been thoroughly examined. Here, we summarize the current understanding of the association between periostin and TGFβ and BMP ligands, and discuss the implications of this association in the context of the role of these molecules in heart valve development and valvular homeostasis. Information about hierarchal connections between periostin and TGFβ and BMP ligands in valvulogenesis will increase our understanding of the pathogenesis, progression, and medical treatment of human valve diseases. KEYWORDS: periostin, transforming growth factor beta, TGFβ, bone morphogenetic protein, BMP, heart development, heart valves, Marfan syndrome INTRODUCTION About one-fourth of patients with congenital heart disease, which affects approximately 5% of live births, have structural anomalies of one or more heart valves[1,2]. Isolated bicuspid aortic valve and congenital polyvalvular disease represent major congenital cardiac valve malformations[3,4,5]. -

Periostin Induces Proliferation of Differentiated Cardiomyocytes and Promotes Cardiac Repair
ARTICLES Periostin induces proliferation of differentiated cardiomyocytes and promotes cardiac repair Bernhard Ku¨hn1, Federica del Monte2, Roger J Hajjar2,4, Yuh-Shin Chang3, Djamel Lebeche2,4, Shima Arab1 & Mark T Keating1,4 Adult mammalian hearts respond to injury with scar formation and not with cardiomyocyte proliferation, the cellular basis of regeneration. Although cardiogenic progenitor cells may maintain myocardial turnover, they do not give rise to a robust regenerative response. Here we show that extracellular periostin induced reentry of differentiated mammalian cardiomyocytes into the cell cycle. Periostin stimulated mononucleated cardiomyocytes to go through the full mitotic cell cycle. Periostin activated aV, b1, b3 and b5 integrins located in the cardiomyocyte cell membrane. Activation of phosphatidylinositol-3-OH kinase was required for periostin- http://www.nature.com/naturemedicine induced reentry of cardiomyocytes into the cell cycle and was sufficient for cell-cycle reentry in the absence of periostin. After myocardial infarction, periostin-induced cardiomyocyte cell-cycle reentry and mitosis were associated with improved ventricular remodeling and myocardial function, reduced fibrosis and infarct size, and increased angiogenesis. Thus, periostin and the pathway that it regulates may provide a target for innovative strategies to treat heart failure. Differentiated cardiomyocytes carry the pump function of the heart. hypertrophic cardiomyopathy16. By contrast, liposomal gene delivery Loss of cardiomyocytes, which occurs, for example, after a large of periostin leads to dilative cardiomyopathy without hypertrophy17. myocardial infarction, typically results in heart failure. Observations Here we have investigated whether recombinant periostin, delivered spanning the twentieth century show that some cardiomyocytes in the through the cardiac extracellular matrix, can increase cardiomyocyte adult heart undergo DNA synthesis and mitosis1,2.