Offshore and Multi-Use Aquaculture with Extractive Species: Seaweeds and Bivalves
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Diversity of Farmed Seafood Across Canada
Contents Report Highlights....................................................................................................................... 4 Introduction .............................................................................................................................. 6 A Diverse and Prosperous Sector .............................................................................................. 7 Production and Revenues Fell in 2019 .............................................................................. 8 Trade ................................................................................................................................. 9 Diversity of Farmed Seafood Across Canada .................................................................. 10 Delivering Economic Benefits to All Canadians ............................................................... 15 An Environmentally Sustainable Sector .................................................................................. 16 Maintaining Animal Health and Welfare ........................................................................ 16 Using Resources Efficiently ............................................................................................. 18 Maintaining Healthy and Productive Ecosystems ........................................................... 23 A Socially Responsible Sector .................................................................................................. 25 Ensuring Safe and Healthy Products .............................................................................. -

Recirculating Aquaculture Systems in China-Current Application And
quac d A ul n tu a r e s e J i o r u e r h n Ying et al., Fish Aquac J 2015, 6:3 s i a F l Fisheries and Aquaculture Journal DOI: 10.4172/2150-3508.1000134 ISSN: 2150-3508 ResearchCommentary Article OpenOpen Access Access Recirculating Aquaculture Systems in China-Current Application and Prospects Liu Ying, Liu Baoliang, Shi Ce and Sun Guoxiang Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, China For the past 20 years, aquaculture has seen a worldwide expansion RAS with independent intellectual property rights achieved from the and is the fastest growing food-producing sector in the world, with an experimental stage gradually turns to industrialization, large-scale average annual growth rate of 6-8%. World aquaculture has grown popularization and application. tremendously during the last sixty years from a production of less than a million tonne in the early 1950s to 90.43 million tonnes by 2012. Of Integrative stage (2007~2011): China has made considerable this production, 66.63 million tonnes, or 73.68%, was fish. Aquaculture progress in research and application of marine industrial aquaculture, is now fully comparable to marine capture fisheries when measured by and established the suitable development mode of China’s RAS. volume of output on global scale. The contribution from aquaculture During this period, many species, such as grouper, half-smooth to the world total fish production of capture and aquaculture in 2012 tongue sole, fugu, abalone, and sea cucumber were firstly cultured in reached 42.2%, up from 25.7% in 2000. -

Canadian Aquaculture
Presented by Canadian agencies and organizations currently undertaking aquaculture research in Canada CANADIAN AQUACULTURE R&D REVIEW Includes 150 summaries of recent research projects on salmon, trout, charr, oysters, mussels, marine species plus special full length features on completed projects across the country. CANADIAN AQUACULTURE R&D REVIEW Bridging research, development CONTENTS and commercialisation Improving awareness of aquaculture R&D key components are a new internal DFO FINFISH - FRESHWATER ......................3 activities in Canada and increasing transfer of Program for Regulatory Research (PARR) and knowledge and technology to the aquaculture core funding for the Centre for Integrated sector has been the goal of the aquaculture Aquaculture Science, a DFO virtual Centre of FINFISH - SALMON .............................9 R&D review since its inception in 2004. It grew Expertise based in St. Andrews, NB that focuses out of efforts by the federal and provincial on ecosystem-based approaches. The objective governments to improve aquaculture R&D of AIMAP is to improve the competitiveness FINFISH - MARINE ............................15 coordination and communication in Canada. of the Canadian aquaculture industry by This third bi-annual edition continues to encouraging an aquaculture sector that build on the success of the first two editions. It continuously develops and adopts innovative POLYCULTURE ..................................18 summarises about 150 R&D projects that have technologies and management techniques been -

Preventing Salmon Escapes from Aquaculture in Canada and the USA: Limited International Coordinates, Divergent Regulatory Currents and Possible Future Courses
Schulich School of Law, Dalhousie University Schulich Law Scholars Articles, Book Chapters, & Blogs Faculty Scholarship 2007 Preventing Salmon Escapes from Aquaculture in Canada and the USA: Limited International Coordinates, Divergent Regulatory Currents and Possible Future Courses David VanderZwaag Tricia Barry Follow this and additional works at: https://digitalcommons.schulichlaw.dal.ca/scholarly_works Part of the Environmental Law Commons RECIEL 16 (1) 2007. ISSN 0962 8797 BlackwellPREVENTINGORIGINAL Publishing ARTICLE SALMON Ltd ESCAPES FROM AQUACULTURE IN CANADAPreventing and THE USA Salmon Escapes from Aquaculture in Canada and the USA: Limited International Coordinates, Divergent Regulatory Currents and Possible Future Courses Tricia K. Barry and David L. VanderZwaag Following an introductory review of the continuing November 2005, tens of thousands of mature salmon problem of salmon escaping from aquaculture opera- escaped in New Brunswick.4 tions along the Atlantic and Pacific coasts of North America, and the considerable uncertainties over Preventing escapes has been a challenge because of ecological impacts, this article examines the law and the multiple ways that releases can occur. Among the policy context for preventing escapes from three per- causes of escapes are poor net maintenance, storm spectives. First, the limited guidance for addressing damage, accidental losses during transfers, tearing of aquaculture escapes under existing global and regional nets by boat collisions or predators, and vandalism.5 agreements/arrangements -

Offshore Aquaculture in the United States: Economic Considerations, Implications & Opportunities
Offshore Aquaculture in the United States: Economic Considerations, Implications & Opportunities July 2008 U.S. Department of Commerce National Oceanic & Atmospheric Administration Silver Spring, Maryland NOAA Technical Memorandum NMFS F/SPO-103 You may download an electronic version of this report from: http://aquaculture.noaa.gov This document should be cited as follows: Rubino, Michael (editor). 2008. Offshore Aquaculture in the United States: Economic Considerations, Implications & Opportunities. U.S. Department of Commerce; Silver Spring, MD; USA. NOAA Technical Memorandum NMFS F/SPO-103. 263 pages. For more information: NOAA Aquaculture Program 1315 East-West Hwy. SSMC #3 – Room 13117 Silver Spring MD 20910 (301) 713-9079 E-mail: [email protected] Website: http://aquaculture.noaa.gov Offshore Aquaculture in the United States: Economic Considerations, Implications & Opportunities Prepared by the NOAA Aquaculture Program From technical contributions by James L. Anderson, John Forster, Di Jin, James E. Kirkley, Gunnar Knapp, Colin E. Nash, Michael Rubino, Gina L. Shamshak, Diego Valderrama NOAA Aquaculture Program 1315 East-West Hwy. SSMC #3 – Room 13117 Silver Spring MD 20910 July 2008 U.S. DEPARTMENT OF COMMERCE Carlos M. Gutierrez, Secretary NATIONAL OCEANIC & ATMOSPHERIC ADMINISTRATION Vice Admiral Conrad C. Lautenbacher, Jr. USN (Ret.), Administrator NATIONAL MARINE FISHERIES SERVICE James Balsiger, Assistant Administrator for Fisheries This page intentionally left blank. TABLE OF CONTENTS Chapter 1: Introduction …………………………………………………………….. 1 Michael Rubino Chapter 2: Economic Potential for U.S. Offshore Aquaculture: An Analytical Approach ………………………………………………. 15 Gunnar Knapp Chapter 3: Emerging Technologies in Marine Aquaculture ……………………….. 51 John Forster Chapter 4: Future Aquaculture Feeds and Feed Costs: The Role of Fish Meal and Fish Oil …………………………………… 73 Gina Shamshak & James Anderson Chapter 5: Lessons from the Development of the U.S. -

AQUACULTURE INDUSTRY and GOVERNANCE in CANADA Standing Senate Committee on Fisheries and Oceans
SBK>QB SK>Q CANADA VOLUME ONE – AQUACULTURE INDUSTRY AND GOVERNANCE IN CANADA Standing Senate Committee on Fisheries and Oceans The Honourable Fabian Manning Chair The Honourable Elizabeth Hubley Deputy Chair July 2015 For more information please contact us: by email: [email protected] by phone: (613) 990-0088 toll-free: 1-800-267-7362 by mail: The Standing Senate Committee on Fisheries and Oceans Senate, Ottawa, Ontario, Canada, K1A 0A4 This report can be downloaded at: www.senate-senat.ca/pofo.asp The Senate of Canada is on Twitter: @SenateCA, follow the committee using the hashtag #POFO Ce rapport est également offert en français. MEMBERS Senators who participated in this study: The Honourable The Honourable Fabian Manning, Elizabeth Hubley, Chair Deputy Chair The Honourable Senators: Sandra Thomas Johnson George Baker M. Lovelace Don Meredith Jim Munson McInnis Nicholas Nancy Greene Carolyn Stewart Rose-May Poirier David M. Wells Raine Olsen Volume 1 – Aquaculture Industry and Governance in Canada i The Committee would like to recognize the following Honourable Senators who are no longer serving members of the Committee whose contribution to the study was invaluable. Tobias C. Lynn Beyak Enverga Jr. Ex-officio members of the Committee: The Honourable Senators Claude Carignan, P.C., (or Yonah Martin) and James S. Cowan (or Joan Fraser). Other Senators who have participated from time to time in this study: The Honourable Senators: Batters, Demers, Fortin-Duplessis, Lang, McIntyre, Mercer, Plett, Tannas. Parliamentary Information and -

Gulf Council Aquaculture Faqs
Gulf of Mexico Fishery Management Council Aquaculture Fishery Management Plan Frequently Asked Questions What is offshore aquaculture? Offshore aquaculture is the rearing of aquatic organisms in controlled environments (e.g., cages or net pens) in federally managed areas of the ocean. Federally managed areas of the Gulf of Mexico begin where state jurisdiction ends and extend 200 miles offshore, to the outer limit of the U.S. Exclusive Economic Zone (EEZ). Why conduct aquaculture offshore? Offshore aquaculture is desirable for several reasons. First, there are fewer competing uses (e.g., fishing and recreation) farther from shore. Second, the deeper water makes it a desirable location with more stable water quality characteristics for rearing fish and shellfish. The stronger waterflows offshore also mitigate environmental effects such as nutrient and organic loading. Are there currently any offshore aquaculture operations in federal waters of the United States? Currently there are no commercial finfish offshore aquaculture operations in U.S. federal waters. There are currently 25 permit holders for live rock aquaculture in the EEZ. There are also several aquaculture operations conducting research and commercial production in state waters, off the coasts of California, New Hampshire, Hawaii, Washington, Maine, and Florida. Why did the Gulf of Mexico Fishery Management Council develop a Fishery Management Plan (FMP) for regulating offshore marine aquaculture in the Gulf of Mexico? The current Federal permitting process for offshore aquaculture is of limited duration and is not intended for the large-scale production of fish, making commercial aquaculture in federal waters impracticable at this time. Offshore aquaculture could help meet consumers’ growing demand for seafood with high quality local supply, create jobs in coastal communities, help maintain working waterfronts, and reduce the nation’s dependence on seafood imports. -

Canadian Fisheries &
Canadian FISHERIES & A Sector Strategy led by Genome AQUACULTURE Atlantic and Genome British Columbia, with support from regional Genome Centres across Canada How genomics can address and funded by Genome Canada. sector challenges August 2013 Genomics* is the science that aims to decipher Genome Canada and the six regional Genome Centres and understand the entire genetic information of across the country are working to harness the transfor- an organism (i.e. microorganisms, plants, animals mative power of genomics to deliver social and and humans) encoded in DNA and corresponding economic benefits to Canadians. complements such as RNA, proteins and metabolites. This paper is one in a series of four sector strategies The knowledge and innovations emerging from funded by Genome Canada and co-led by the Genome this field are finding solutions to complex biological Centres. They include: Agri-Food, Energy and Mining, challenges, while at the same time raising questions Fisheries and Aquaculture, and Forestry. Each strategy, of societal and economic importance. developed in consultation with sector stakeholders, maps out how the sector can further leverage the Genomics has already brought huge economic and transformative power of genomics, and related disci- societal gains to Canadians through better healthcare, plines, to its advantage. improving food quality, safety and production and protecting our environment and natural resources. Given Canada’s footprint in these key natural resource sectors, the time is ripe for our industries to take full Looking ahead, genomics will be the foundation of advantage of the power and promise of genomics. Canada's growing bio-economy (all economic activity derived from life science-based research), which is *Broadly speaking, our definition of genomics includes estimated to be responsible for some 2.25 per cent related disciplines such as bioinformatics, epigenomics, of GDP, or about $38 billion, by 2017. -

Canadian Aquaculture Statistics
Catalogue no. 23-222-X Aquaculture Statistics 2015 How to obtain more information For information about this product or the wide range of services and data available from Statistics Canada, visit our website, www.statcan.gc.ca. You can also contact us by e-mail at [email protected] telephone, from Monday to Friday, 8:30 a.m. to 4:30 p.m., at the following toll-free numbers: • Statistical Information Service 1-800-263-1136 • National telecommunications device for the hearing impaired 1-800-363-7629 • Fax line 1-877-287-4369 Depository Services Program • Inquiries line 1-800-635-7943 • Fax line 1-800-565-7757 To access this product This product, Catalogue no. 23-222-X, is available free in electronic format. To obtain a single issue, visit our website, www.statcan.gc.ca and browse by “Key resource” > “Publications.” Standards of service to the public Statistics Canada is committed to serving its clients in a prompt, reliable and courteous manner. To this end, this agency has developed standards of service that its employees observe. To obtain a copy of these service standards, please contact Statistics Canada toll-free at 1-800-263-1136. The service standards are also published at www.statcan.gc.ca under “Contact us” > “Standards of service to the public.” Statistics Canada Agriculture Division Commodities Section Aquaculture Statistics 2015 Published by authority of the Minister responsible for Statistics Canada © Minister of Industry, 2016 All rights reserved. Use of this publication is governed by the Statistics Canada Open License Agreement. http://www.statcan.gc.ca/reference/licence-eng.html November 2016 Catalogue no. -

18 TILAPIA CULTURE in MAINLAND CHINA Lai Qiuming1 and Yang Yi2
TILAPIA CULTURE IN MAINLAND CHINA Lai Qiuming1 and Yang Yi2 1College of Aquaculture, Hainan University, Haikou, Hainan, China 2Aquaculture and Aquatic Resources Management, School of Environment, Resources and Development, Asian Institute of Technology, Pathum Thani, Thailand Abstract The first tilapia species introduced to mainland China is Mozambique tilapia (Oreochromis mossambicus) from Vietnam in 1957. Since then, several tilapia species such as blue tilapia (O. aureus) and different strains of Nile tilapia (O. niloticus) have been introduced to China from different places. Tilapia culture in China started in early 1960s, but was not popular until early 1980s. Since then, tilapia culture has been expanded rapidly in response to the introduction of new strains, success in all-male tilapia production and improvement in both nursing and grow-out technologies. Tilapia production increased from 18,100 metric tons in 1984 to 706,585 metric tons in 2002, with an average annual growth rate of 25%. Considering the low seafood especially tilapia consumption per capita, large domestic markets and technology improvements, there is still a huge potential for further expansion of tilapia culture in China. Introduction There is a growing consensus that tilapias (Oreochromis spp.) can become one of the world’s most important cultured fishes (FAO, 1980). Since 1980s, culture of tilapias has been quickly expanded in many parts of the world, especially in China. The attributes which makes tilapias especially Nile tilapia (O. niloticus) so suitable for fish farming are their general hardiness, great tolerance to adverse environmental conditions, ease of breeding, rapid growth rate, ability to efficiently convert organic and domestic wastes into high quality protein, and good taste (Stickney et al., 1979; Balarin and Haller, 1982; Pullin and Lowe- McConnell, 1982). -
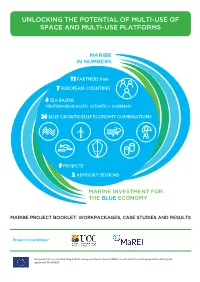
Maribe in Numbersin Numbers
UNLOCKING THE POTENTIAL OF MULTI-USE OF SPACE AND MULTI-USE PLATFORMS MARIBEMARIBE IN NUMBERSIN NUMBERS 11 PARTNERS from 7 EUROPEAN COUNTRIES 4 SEA BASINS MEDITERRANEAN, BALTIC, ALTANTIC & CARIBBEAN 24 BLUE GROWTH/BLUE ECONOMY COMBINATIONS 9 PROJECTS 2 ADVISORY SESSIONS MARINEMARINE INVESTMENT INVESTMENT FOR FORTHE BLUETHE BLUE ECONOMY ECONOMY MARIBE PROJECT BOOKLET: WORKPACKAGES, CASE STUDIES AND RESULTS Project coordinator This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 652629 UNLOCKING THE POTENTIAL OF MULTI-USE UNLOCKING THE POTENTIAL OF MULTI-USE OF SPACE AND MULTI-USE PLATFORMS OF SPACE AND MULTI-USE PLATFORMS Contents Partner Organisations and List of Abbreviations ................................................................................ .2 Introduction ....................................................................................................................................... 3 WP Summaries ................................................................................................................................... 6 Work Package -‐ 4 Socio-‐Economic Trends and EU Policy in the Offshore Economy ..................... 6 WP 5 -‐ Te chnical and Non-‐technical Challenges, Regional and Sectoral......................... ............... 9 WP 6 -‐ In vestment community consultation and commitment....................................... ............. 10 WP 7 -‐ Business Model Mapping and Assessment...................................................................... -

System Analysis of a High-Value Aquaponics Innovation in Ontario
System analysis of a high-value aquaponics innovation in Ontario By Mashiur M. Rahman A Major Research Paper Presented to The University of Guelph In partial fulfillment of requirements for the degree of Masters of Science in Capacity Development and Extension Guelph, Ontario, Canada © Mashiur Rahman, August 2017 ABSTRACT System analysis of a high-value aquaponics innovation in Ontario Mashiur M. Rahman Advisor: University of Guelph, 2016 Dr. Helen Hambly Odame This research paper examines the system of aquaponic farming for sustainable food production in Ontario, Canada. Currently, aquaponics is becoming quite adaptable and amenable globally for community-led social enterprises and capacity building. The literature review discusses stakeholder’s perceptions and awareness, a SWOT (Strengths, Weaknesses, Opportunities, and Threats) analysis of aquaponics farming and its commercial viability in Ontario, Canada, and two commercial aquaponics case studies in Ontario. The objective of this paper is to explore the sustainability and feasibility of aquaponic farming in Ontario for the purpose of improving food security. Sustainable aquaponic operations rely on careful analysis, planning, and management of resources. The case studies demonstrate that aquaponic operations in Ontario are social, environmentally and economically viable. Ultimately, community-based sustainable aquaponic farming will be a viable solution for food security for an increasing population globally. This study is significant because it describes the sustainability of commercial aquaponic farming in Ontario. However, further research is needed to help minimize energy costs and provide economically affordable fish feed to ensure profitability in aquaponics. Key words: aquaponics, fish, tilapia, closed-loop systems, economic development, sustainability, recirculating aquaculture system, hydroponics 2 Acknowledgements First and foremost, I would like to extend my deep gratitude to my advisor Dr.