SPECTROPHOTOMETRIC DETERMINATION of CEPHALEXIN USING NINHYDRIN REAGENT in TABLET DOSAGE FORM Patel Satish A*, Patel Natavarlal J
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

River Inundation and Hazard Mapping a Case Study of North Zone –Surat City
Patel Chandresh G, Dr.P.J. Gundaliya . /International Journal of New Technologies in Science and Engineering Vol. 2, Issue 2, Aug 2015, ISSN 2349-0780 River Inundation and Hazard Mapping a Case Study of North Zone –Surat City Patel Chandresh G, Assistant Professor, Ganpat University, Kherva Dr.P.J. Gundaliya, Associate Professor, LDCE, Ahmedabad Abstract: New technologies, such as Geographical Information System (GIS) and Remote Sensing (RS) are helping the researchers to create accurate floodplain maps with improved efficiency. This paper illustrates generation of Digital Elevation Model (DEM) and GIS applications for developing floodplain models of North Zone of Surat City, fastest growing city of Gujarat state, India. Flood event occurred in 2006 and discharge observed near Nehru Bridge is taken as reference to workout probable submergence depth of different area lying in North Zone of Surat city. Based on flood frequency analysis of the study area flood inundation map for flood return period of 30, 32 (Worst flood event-2006) and 35 years (future flood event) are generated in GIS environment. Keywords: DEM, Flood, North Zone, River I. INTRODUCTION Flooding is one of the serious natural hazards in the world [1] ,and it accounts for 40% of all deaths caused by natural disasters, with most flood events occurring in developing, tropical regions [2] .Floods are of many types including flash flooding [3] , flooding due to rising ground water [4] , coastal flooding [5] and flooding due to the opening or breaking of a dam or reservoir [6] .Floods resulting from excessive rainfall within a short period of time and consequent high river discharge damage crops and infrastructure. -
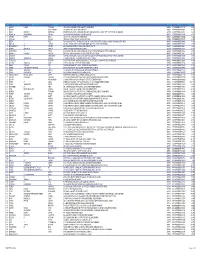
Section 124- Unpaid and Unclaimed Dividend
Sr No First Name Middle Name Last Name Address Pincode Folio Amount 1 ASHOK KUMAR GOLCHHA 305 ASHOKA CHAMBERS ADARSHNAGAR HYDERABAD 500063 0000000000B9A0011390 36.00 2 ADAMALI ABDULLABHOY 20, SUKEAS LANE, 3RD FLOOR, KOLKATA 700001 0000000000B9A0050954 150.00 3 AMAR MANOHAR MOTIWALA DR MOTIWALA'S CLINIC, SUNDARAM BUILDING VIKRAM SARABHAI MARG, OPP POLYTECHNIC AHMEDABAD 380015 0000000000B9A0102113 12.00 4 AMRATLAL BHAGWANDAS GANDHI 14 GULABPARK NEAR BASANT CINEMA CHEMBUR 400074 0000000000B9A0102806 30.00 5 ARVIND KUMAR DESAI H NO 2-1-563/2 NALLAKUNTA HYDERABAD 500044 0000000000B9A0106500 30.00 6 BIBISHAB S PATHAN 1005 DENA TOWER OPP ADUJAN PATIYA SURAT 395009 0000000000B9B0007570 144.00 7 BEENA DAVE 703 KRISHNA APT NEXT TO POISAR DEPOT OPP OUR LADY REMEDY SCHOOL S V ROAD, KANDIVILI (W) MUMBAI 400067 0000000000B9B0009430 30.00 8 BABULAL S LADHANI 9 ABDUL REHMAN STREET 3RD FLOOR ROOM NO 62 YUSUF BUILDING MUMBAI 400003 0000000000B9B0100587 30.00 9 BHAGWANDAS Z BAPHNA MAIN ROAD DAHANU DIST THANA W RLY MAHARASHTRA 401601 0000000000B9B0102431 48.00 10 BHARAT MOHANLAL VADALIA MAHADEVIA ROAD MANAVADAR GUJARAT 362630 0000000000B9B0103101 60.00 11 BHARATBHAI R PATEL 45 KRISHNA PARK SOC JASODA NAGAR RD NR GAUR NO KUVO PO GIDC VATVA AHMEDABAD 382445 0000000000B9B0103233 48.00 12 BHARATI PRAKASH HINDUJA 505 A NEEL KANTH 98 MARINE DRIVE P O BOX NO 2397 MUMBAI 400002 0000000000B9B0103411 60.00 13 BHASKAR SUBRAMANY FLAT NO 7 3RD FLOOR 41 SEA LAND CO OP HSG SOCIETY OPP HOTEL PRESIDENT CUFFE PARADE MUMBAI 400005 0000000000B9B0103985 96.00 14 BHASKER CHAMPAKLAL -

International Journal of Innovative Technology and Exploring Engineering
International Journal of Innovative Technology and Exploring Engineering ISSN : 2278 - 3075 Website: www.ijitee.org Volume-9 Issue-8, JUNE 2020 Published by: Blue Eyes Intelligence Engineering and Sciences Publication xploring En E gi d ne an e r y in g g lo o n h c e T IjItEe e I n v i t t e E a X r v P N n o L O a O I n T t R A i o I V n N O I G N n IN f a o l l J a o r n u www.ijitee.org Exploring Innovation Editor-In-Chief Dr. Shiv Kumar Ph.D. (CSE), M.Tech. (IT, Honors), B.Tech. (IT), Senior Member of IEEE, Member of the Elsevier Advisory Panel CEO, Blue Eyes Intelligence Engineering and Sciences Publication (BEIESP), Bhopal (MP), India Associate Editor-In-Chief Dr. Takialddin Al Smadi Professor, Department of Communication and Electronics, Jerash Universtiy, Jerash, Jordan Dr. Vo Quang Minh Senior Lecturer and Head, Department of Land Resources, College of Environment and Natural Resources (CENRes), Can Tho City, Vietnam. Dr. Stamatis Papadakis Lecturer, Department of Preschool Education, University of Crete, Greece. Dr. Ali OTHMAN Al Janaby Lecturer, Department of Communications Engineering, College of Electronics Engineering University of Ninevah, Iraq. Dr. Rabiul Ahasan Professor, Department of Industrial Engineering, King Saud University, Saudi Arabia. Dr. Hakimjon Zaynidinov Professor and Head, Department of Computer Science, Tashkent University of Information Technologies, Uzbekistan. Prof. MPS Chawla Ex-Chairman, IEEE MP Sub-Section, India, Professor-Incharge (head)-Library, Associate Professor in Electrical Engineering, G.S. -

University Aishe Code
University Aishe Code Aishe Srno Code University Name 1 U-0122 Ahmedabad University (Id: U-0122) 2 U-0830 ANANT NATIONAL UNIVERSITY (Id: U-0830) 3 U-0125 Carlox Teachers University, Ahmedabad (Id: U-0125) Centre for Environmental Planning and Technology University, Ahmedabad (Id: U- 4 U-0127 0127) 5 U-0131 Dr. Babasaheb Ambedkar Open University, Ahmedabad (Id: U-0131) 6 U-0775 GLS UNIVERSITY (Id: U-0775) 7 U-0135 Gujarat Technological University, Ahmedbabd (Id: U-0135) 8 U-0136 Gujarat University, Ahmedabad (Id: U-0136) 9 U-0817 GUJARAT UNIVERSITY OF TRANSPLANTATION SCIENCES (Id: U-0817) 10 U-0137 Gujarat Vidyapith, Ahmedabad (Id: U-0137) 11 U-0139 Indian Institute of Technology, Gandhinagar (Id: U-0139) 12 U-0922 Indrashil University (Id: U-0922) INSTITUTE OF INFRASTRUCTURE TECHNOLOGY RESEARCH AND MANAGEMENT 13 U-0765 (IITRAM) (Id: U-0765) 14 U-0734 LAKULISH YOGA UNIVERSITY, AHMEDABAD (Id: U-0734) 15 U-0146 Nirma University, Ahmedabad (Id: U-0146) 16 U-0790 RAI UNIVERSITY (Id: U-0790) 17 U-0595 Rakshashakti University, Gujarat (Id: U-0595) 18 U-0123 Anand Agricultural University, Anand (Id: U-0123) 19 U-0128 Charotar University of Science & Technology, Anand (Id: U-0128) 20 U-0148 Sardar Patel University, Vallabh Vidyanagar (Id: U-0148) 21 U-0150 Sardarkrushinagar Dantiwada Agricultural University, Banaskantha (Id: U-0150) 22 U-0124 Maharaja Krishnakumarsinhji Bhavnagar University (Id: U-0124) 23 U-0126 Central Univeristy of Gujarat, Gandhinagar (Id: U-0126) 24 U-0594 Children University, Gandhinagar (Id: U-0594) Dhirubhai Ambani Institute -

Name of Regional Directorate of NSS- Ahmedabad State-Gujarat
Name of Regional Directorate of NSS- Ahmedabad State-Gujarat Regional Director Name Address Email ID Telephone/Mobile/Landline Number Sh. GirdharUpadhyay Regional Directorate of NSS, [email protected] 079-26565988 2ndfloor,PatnagarYojnaBhavan, 7999894816 Ellis bridge ,Ahmedabad-380006 Secretary, dealing with NSS Name Address E Mail ID Telephone/Mobile Number Shri S.J. Haider , IAS Block No.-5, [email protected] 079-23251301/303 Principle Secretary 7th,Floor Fax 07923251325 Education Department Sachivalaya, Government of Gujarat Gandhinagar,Gujarat State NSS Officer/Officer acting as SNO Name Address E Mail ID Telephone/Mobile Number ShriYashwant Kumar HPatel Commissionerate of Higher Education [email protected] 9427685870 State NSS Officer Govt. of Gujarat, Old Sachivalaya 079-23253993 Block No.12/2,Dr. Jivraj Mehta Bhavan, Gandhinagar ,Gujarat Programme Coordinator, NSS Name Address E Mail ID Telephone/Mobile Number 1 Dr Shreedhar Nimavat Veer Narmad South Gujarat [email protected], 0261-2203039 Programme co-ordinator, NSS University, [email protected] 8780077566 Veer Narmad South Gujarat University Campus, University, Surat UdhnaMagdalla Road, Surat-395007 2 Dr. J. D. Damor Hemchandracharya [email protected] 02766-230743,Ext.316 Hemchandrcharya North North Gujarat University, 9925046204, 7573010065 Gujarat University, Patan Raj Mahal Road, P.B. No. 21 Patan-384265 3 Dr. N. K. Dobariya Saurashtra University, [email protected] 02812578501 Saurashtra University, Rajkot Kalavad Road, 9687692940 Rajkot-360 005 4 Dr. JagrutiSuvera Sardar Patel University, [email protected] 02692-226823 Sardar Patel University, VallabhVidyanagar, [email protected] 9408507810 VallabhVidyanagar,Anand Dist : Anand- 388120 5 Dr. Arunbhai Gandhi, Gujarat Vidyapith, [email protected] 079-4001630 Gujarat Vidyapeeth, Ashram Road, 9428214260 Ahmedabad Ahmedabad-380014 6 Dr. -

GANDHINAGAR Roll of Registered Graduates As on 12/31/2019 (2020 - 2024)
GUJARAT AYURVED UNIVERSITY - JAMNAGAR Polling Centre : GANDHINAGAR Roll of Registered Graduates as on 12/31/2019 (2020 - 2024) Sr. Name Taluka Renewal Degree No. Address District Comp. No. 1 ACHARYA JAYENDRAKUMAR DEVENDRAPRASADVISNAGAR 2022 B.S.A.M. 54-B, KRUSHANNAGAR SOCIETY, OPP.DHAROI COLONY, TA-VISNAGAR DIST. MEHSANA MEHSANA 10488 DENAP 2 AKHUNJI SADIYA MOHAMMEDHABIB KADI 2020 B.A.M.S. HASA SERI,NEAR MEHSANA BANK, KADI MAHESANA 15822 KADI 3 ANJAN NITINKUMAR PUNAMCHAND GANDHINAGAR 2022 B.A.M.S. PLOT NO.800/1, SECTOR-4-C, GANDHINAGAR GANDHINAGAR 14875 GANDHINAGAR 4 BALSARA TEJENDRAKUMAR RAMESHCHANDRAMAHESANA 2023 B.A.M.S. I/15 SANIDHYA BANGLOWS, AKSHARDHAM TOWNSHIP, OPP.N.G. INT. SCHOOL MAHESANA 8339 MAHESANA 5 BAROT ANAND ASHOKBHAI GANDHINAGAR 2023 B.A.M.S. 384/2, SECTOR-3B Gandhinagar 16575 GANDHINAGAR 6 BAROT KARANKUMAR DILIPKUMAR JOTANA 2020 B.A.M.S. AT; SIDOSANA, POST; JAKASANA, TA; JOTANA MAHESANA 15413 JAKASANA 7 BHABHOR BIPINKUMAR VAGHAJIBHAI GANDHINAGAR 2020 B.A.M.S. BLOCK NO. 155/5, J-2 TYPE, SECTOR-7, GANDHINAGAR GANDHINAGAR 15272 GANDHINAGAR 8 BHANDERI SANJAYBHAI PRAKASHBHAI GANDHINAGAR 2022 B.A.M.S. 400-GAYATRINAGAR SOCIETY, SECTOR-27 POST-SECTOR-28 GANDHINAGAR 14876 GANDHINAGAR 9 BHATT RAKESH NAVNEETLAL GANDHINAGAR 2022 B.A.M.S. ANANDVATIKA, 152/2, SECTOR-22 GANDHINAGAR 11845 GANDHINAGAR 10 BHATT SUKRUTI GIRISHBHA MEHSANA 2023 B.A.M.S. 21- SHYAMVIHAR BUNGLOWS-2, RADHANPUR ROAD, BEHIND SAI KRISHNA HOSPITAL MEHSANA 10377 MEHSANA 11 BHAVSAR JIGAR SHASHIKANT MEHASANA 2023 B.A.M.S. B/17 BHAKUNTAL BUNGLOWS, NR PATIDAR NAGAR SOCIETY, MEHSANA MEHASANA 2494 MEHASANA 12 BHILOCHA ASHVINKUMAR GANPATRAMGANDHINAGAR 2023 B.S.A.M. -

ANSWERED ON:28.07.2016 Marine Manpower Jardosh Smt
GOVERNMENT OF INDIA SHIPPING LOK SABHA UNSTARRED QUESTION NO:1941 ANSWERED ON:28.07.2016 Marine Manpower Jardosh Smt. Darshana Vikram Will the Minister of SHIPPING be pleased to state: (a) whether the Government has assessed the requirements of marine manpower in the country and if so, the details and the shortfall thereof; (b) the details of marine engineering universities/colleges functioning in the country, State-wise; (c) whether the Government proposes to set up more such universities/colleges/ institutes in the country; (d) if so, the details thereof and the fund allocated for the purpose, State/UT-wise; and (e) the time by which these are likely to be set up? Answer MINISTER OF STATE IN THE MINISTRY OF SHIPPING (SHRI PON. RADHAKRISHNAN) (a) The marine manpower requirement depends upon the market forces of demand & supply of the global shipping industry. Against the approved capacity of 15524 seats in pre-sea courses in maritime training institutions, only 10664 in 2013, 11327 in 2014 and 10425 in 2015 could be filled up. (b) A total of 30 maritime training institutes approved by the Directorate General of Shipping, Government of India conduct marine engineering courses in the country. These institutes are affiliated to fourteen different Universities in the country. A list of such institutes with their affiliation details is annexed. (c) No, Madam. There is no such proposal. (d)&(e):Do not arise in view of (c) above. <pre> Annexure List of Maritime Training Institutes Conducting Marine Engineering Course S NAME OF THE MARITIME INSTITUTE & ADDRESS UNIVERSITY Andhra Pradesh 1 Praveenya Institute of Marine Engineering & Maritime Studies, Opp. -

Ganpatuni Act05
GUJARAT ACT NO. 19 OF 2005. THE GANPAT UNIVERSITY ACT, 2005. SECTIONS. PAGE NO. CONTENTS CHAPTER I PRELIMINARY 1. Short title and Commencement. 3 2. Definitions. 4 CHAPTER II UNIVERSITY 3. Establishment and incorporation of University. 4 4. Objects of University. 6 5. University open to all irrespective of sex, religion, 6 class, creed or opinion. 6. Powers and functions of University. 6 CHAPTER III AUTHORITIES OF UNIVERSITY 7. Patron-in-Chief, President and his powers. 9 8. Authorities of University. 9 9. Officers of University. 10 10. Board of Governors. 10 11. Chairman of the Board. 10 12. Powers and functions of the Board. 11 13. Terms of office and vacancies among members of 11 the Board. 14. Academic Council. 12 15. Powers and duties of Academic Council. 12 16. Finance Committee. 13 17. Powers and functions of Finance Committee. 13 18. Director. 13 19. Powers and duties of the Director. 14 20. Registrar. 15 21. The Deans. 15 2 CHAPTER IV FUNDS, ACCOUNTS AND AUDIT 22. Permanent Endowment Fund of the University. 16 23. Payment to University. 16 24. Funds of University. 16 25. Accounts and Audit. 16 26. Pension and Provident Fund. 17 CHAPTER V SUPPLEMENTARY PROVISIONS 27. Acts and proceedings not to be invalidated by 17 vacancies. 28. Conferment of degrees, diplomas and grant of 17 certificates by University. 29. Returns and information. 17 30. Management of University on dissolution of 18 Foundation. 31. Officers and employees to be public servants. 18 32. Dismissal, removal, reduction and termination of 18 service of staff of University. CHAPTER VI MISCELLANEOUS PROVISIONS 33. -

STATE DISTRICT BRANCH ADDRESS CENTRE IFSC CONTACT1 CONTACT2 CONTACT3 MICR CODE ANDAMAN and NICOBAR ISLAND ANDAMAN Port Blair MB
STATE DISTRICT BRANCH ADDRESS CENTRE IFSC CONTACT1 CONTACT2 CONTACT3 MICR_CODE ANDAMAN AND MB 23, Middle Point, Mrs. Kavitha NICOBAR Port Blair - 744101, Ravi - 03192- ISLAND ANDAMAN Port Blair Andaman PORT BLAIR ICIC0002144 232213/14/15 PARAMES WARA ICICI BANK LTD., RAO OPP. R. T. C. BUS KURAPATI- ANDHRA STAND, 98489 PRADESH ADILABAD ADILABAD ADILABAD.504 001 ADILABAD ICIC0000617 37305- 4-3-168/1, TNGO’S ROAD (CINEMA ROAD ), PADAM HAMEEDPURA CHAND (DWARIKA NAGAR) GUPTA OPP. SRINIVASA 08732- NURSING HOME, 230230; ANDHRA ADILABAD 504001 934788180 PRADESH ADILABAD ADILABAD (A.P>) ADILABAD ICIC0006648 1 ICICI Bank Ltd., Plot No. 91 & 92, Mr. Ramnathpuri Scheme, Satyendra ANDHRA JAIPUR,JHOTWA Jhotwara, Jaipur - Bhatt-141- PRADESH ADILABAD RA 302012, Rajasthan JAIPUR ICIC0006759 3256155 302229057 SUSHIL RAMBAGH PALACE KUMAR JAIPUR,RAM HOTEL RAMBAGH VYAS,0141- ANDHRA BAGH PALACE CIRCLE JAIPUR 3205604,,9 PRADESH ADILABAD HOTEL 302004 JAIPUR ICIC0006778 314661382 ICICI BANK LTD., NO. 12-661, GOKUL COMPLEX, BELLAMPALLY 08736 ROAD, MANCHERIAL, 255232, ANDHRA ADILABAD DIST. 504 MANCHERIY 08736 PRADESH ADILABAD MANCHERIYAL 208 AL ICIC0000618 255234 ICICI BANK LTD., OLD GRAM PANCHAYAT, MUDHOL - RAJESH 504102, TUNGA - ANDHRA ADILABAD DIST., +91 40- PRADESH ADILABAD MUDHOL ANDHRA PRADESH MUDHOL ICIC0002045 41084285 91 9908843335 504229502 ICICI BANK LIMITED PODDUTOOR COMPLEX, D.BO.1-2- 275, (OLD NO 1-2-22 TO 26 ) OPP . BUS SHERRY DEPOT, NIRMAL, JOHN 8942- NIRMAL ADILABAD (DIST), 224213 ANDHRA ANDHRA ANDHRA PRADESH – ,800847763 PRADESH ADILABAD PRADESH 504106 NIRMAL ICIC0001533 2 ICICI BANK LTD, NAVEED MR. SAI RESIDENCY, RAJIV GOPAL ROAD, PATRO ANDHRA ANANTAPUR- 515001 ANANTAPU (08554) - PRADESH ANANTAPUR ANANTPUR ANDHRA PRADESH R ICIC0000439 645033 ICICI BANK LTD., D.NO:12/114 TO 124, PRAMEEL R.S.ROAD, OPP DEVI A P -08559- NURSING HOME, 223943, ANDHRA DHARMAVARAM.515 DHARMAVA 970301725 PRADESH ANANTAPUR DHARMAVARAM 671 RAM ICIC0001034 4 16-337, GUTTI ROAD, GUNTAKAL-DIST. -

District Human Development Report of Mahesana
District Human Development Report MAHESANA Gujarat Social Infrastructure Development Society (GSIDS) General Administration Department (Planning) Government of Gujarat Gujarat Social Infrastructure Development Society (GSIDS) General Administration Department (Planning) Government of Gujarat, Gandhinagar District Human Development Report: MAHESANA Copyright : ©2016 Gujarat Social Infrastructure Development Society (GSIDS), Government of Gujarat Published By : Gujarat Social Infrastructure Development Society (GSIDS) First Published : 2016 All rights reserved. No part of this publication may be reproduced, stored or transmitted in any form by any means without the prior permission from the publisher. This Report does not necessarily reflect the views of the Gujarat Social Infrastructure Development Society (GSIDS). While every care has been taken to reproduce the accurate data, oversights / errors may occur. If found convey it to the Gujarat Social Infrastructure Development Society (GSIDS). The report is prepared by Ganpat University under a tripartite MoU between Member Secretary, Gujarat Social Infrastructure Development Society (GSIDS), District Collector, Mahesana and Principal, V. M. Patel College of Management Studies, Ganpat University. Gujarat Social Infrastructure Development Society (GSIDS) General Administration Department (Planning) Government of Gujarat MESSAGE Human Development is a development paradigm which is beyond mere rise or fall of national incomes. It is about creating an environment where people can develop their full potential and lead productive, creative lives in accordance with their needs and interests. People are the real wealth of nation. Development is thus about expanding the choices people have to lead lives that they value. The District Human Development Report is a Document which gives the present status of Human Development in different talukas of the District. -

Gujarat Water Resources Development Corporation Ltd
GUJARAT WATER RESOURCES DEVELOPMENT CORPORATION LTD. GANDHINAGAR Executive Engineer ( C) Unit-1( G.W.M). Kherva ( Mehsana) ON LINE TENDER Notice No.2 of 2015-16 e-Tenders Notice Name of work :- Tender for Implementation of PINS along with MIS works under micro irrigation system at various G.W.R.D.C.Ltd. Running tubewells which is operated by farmers juth/co.op.society in Mehsana, Patan & Banaskantha Districts. invited by the Executive Engineer (C) Unit-1 (G.W.M.) Opp. Ganpat university campus Kherva-382711 Taluka & District Mehsana Ph.No.02762-286508, 286181 From those suppliers who are registered with the G.G.R.C. Vadodara for MIS Suppliers (i) Date of downloading of Tender Documents and Online submission from Dt.09-07-15 to 07-08-15@ 18.00. Hrs. (ii) Tender Opening on Date 21-.08-15 @ 12.00 Hrs. (iii) Date of submission to group no.BK-02, MH-02 & PT- 02 Ex.Engr (C) Unit-1 (G.W.M.) Kherva by RPAD/ Speed post / Hand Delivery only for EMD, tender fee and other documents from 10-08-15 & 11-08-15 up to 18.00Hrs. The details of the above work will be available on www.nprocure.com , www.gwrdc.gujarat.gov.in and www.statetenders.com Tender will be accepted only by on-line. Tender will not be accepted by R.P.A.D.Speed Post or by Ordinary Post. Executive Engineer (C ) Unit-1, (G.W.M) Kherva (Mehsana) к . кк () -1 (.. .) к , х к - , - 382711 .º -02762-286181/286508 -Ú Ú .2 2015-16 кк () -1, (. -
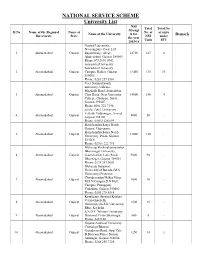
University List.Pdf
NATIONAL SERVICE SCHEME University List NSS Total Total No. Strengt Sl.No Name of the Regional Name of No. of of units Name of the University h for Remark . Directorate State NSS under the year Units SFU 2015-16 Gujarat University, Navrangpura, Near L D 1 Ahemadabad Gujarat Engineering college, 14700 147 4 Ahmedabad, Gujarat 380009 Phone:079 2630 0342 Saurashtra University, Saurashtra University 2 Ahemadabad Gujarat Campus, Rajkot, Gujarat 13450 135 33 360005 Phone: 0281 257 8501 Veer Narmad South university, Udhana - Magdalla Road, Someshwar 3 Ahemadabad Gujarat Char Rasta, Near University 14000 140 4 College, Choryasi, Surat, Gujarat 395007 Phone:0261 222 7146 Sardar Patel University, Vallabh Vidyanagar, Anand, 4 Ahemadabad Gujarat 5000 50 - Gujarat 388120 Phone: 02692 226 899 Hemchandracharya North Gujarat University, Hemchandracharya North 5 Ahemadabad Gujarat 12000 120 - University, Patan, Gujarat 384265 Phone: 02766 222 744 Maharaja Krishnakumarsinhji Bhavanagar University, 6 Ahemadabad Gujarat Gaurishanker Lake Road, 9000 90 - Bhavnagar, Gujarat 364001 Phone: 0278 243 0002 Maharaja Sayajirao University of Baroda,(M.S University) Professor Chandravadan Mehta Marg, 7 Ahemadabad Gujarat 1000 10 - M.S.N Campus,D.N Hall Campus, Pratapgunj, Vadodara, Gujarat 390002 Phone: 0265 278 8814 Krantiguru Shyamji Krishna Verma Kachchh 8 Ahemadabad Gujarat 1500 15 - University,(K.S.K University) Bhuj, Kachchh S.N.D.T Women University, 9 Ahemadabad Gujarat Diamond Circle,Bhavnagar. 800 8 - Phone 2423180 Gujarat Ayurved University, Chanakya Bhavan,