Sustaining Innovation: a Market Driven Pathway
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

1. Cause List of Cases Filed Between 01.01.2018 to 21.03.2020 Shall Not Be Published Till Further Orders
05.04.2021 IN PARTIAL MODIFICATION RELATING TO THE SITTING ARRANGEMENT OF THE HON'BLE JUDGES w.e.f. 05.04.2021, THE COURT NUMBERS ALREADY SHOWN IN THE ADVANCE CAUSE LIST FOR 5th & 6th APRIL FOR THE FOLLOWING HON'BLE JUDGES SHALL NOW BE READ AS UNDER: JUDGES NAME COURT NO. 1. HON'BLE MR. JUSTICE SANJEEV SACHDEVA 7 2. HON'BLE MR. JUSTICE VIBHU BAKHRU 43 3. HON'BLE MR. JUSTICE V. KAMESWAR RAO 14 4. HON'BLE MR. JUSTICE PRATEEK JALAN 16 “All the Advocates/Litigants are informed that in view of the directions dated 09.03.2021 passed by Hon. DB-II in W.P.(C) 2018/2021 and W.P.(C) 2673/2021 half of the cases (starting from the Supplementary List/s) listed for a particular day shall be taken up in the Pre-lunch Session and rest of the cases shall be taken up in the Post-lunch Session. All the Advocates/Litigants may accordingly reach the Court Rooms according to the turn of their case/s in order to curtail the number of people in court premises at the same time.” NOTE 1. CAUSE LIST OF CASES FILED BETWEEN 01.01.2018 TO 21.03.2020 SHALL NOT BE PUBLISHED TILL FURTHER ORDERS. HIGH COURT OF DELHI: NEW DELHI No. 384/RG/DHC/2020 DATED: 19.3.2021 OFFICE ORDER HON'BLE ADMINISTRATIVE AND GENERAL SUPERVISION COMMITTEE IN ITS MEETING HELD ON 19.03.2021 HAS BEEN PLEASED TO RESOLVE THAT HENCEFORTH THIS COURT SHALL PERMIT HYBRID/VIDEO CONFERENCE HEARING WHERE A REQUEST TO THIS EFFECT IS MADE BY ANY OF THE PARTIES AND/OR THEIR COUNSEL. -

Dividend and Shares Liable to Be Transferred to IEPF / IEPFA
THE WATERBASE LIMITED List of Shareholders whose Unclaimed Dividend and Shares Liable to be Transferred to IEPF / IEPFA Dividend Dividend Dividend Dividend Dividend Shares Liable to Folio / DP_Client ID Name of Shareholder (Interim - (Final - 2014- (Final - 2016- (Final - 2017- (Final - 2018- be Transferred 2014-15) 15) 17) 18) 19) to IEPFA Rs. Nos '00000003 DUTT S K 10 5 10 15 15 10 '00000006 DASS H C 10 5 10 15 15 10 '00000103 SANJAY 200 100 200 300 300 200 '00000109 KEMAYA LALL KIDWAI 3000 1500 3000 4500 4500 3000 '00000110 NAINA LALL 1800 900 1800 2700 2700 1800 '00000111 SUNIL KUMAR G 400 200 400 600 600 400 '00000115 VIJAYALAKSHMI G 600 300 600 900 900 600 '00000117 MURARISETTY NAGARAJA LAKSHMI 200 100 200 300 150 100 '00000118 MURARISETTY PULLAIAH 600 300 600 900 750 500 '00000125 SUJATHA KISHORE 300 150 300 450 300 200 '00000127 VIJI SAMPATH 100 50 100 150 150 100 '00000130 MALLIKA AHIRRAO 500 250 500 750 750 500 '00000138 ANIL KUMAR SEELAM 300 150 300 450 450 300 '00000139 BHAGYA LAKSHMI SEELAM 500 250 500 750 750 500 '00000141 MALTI MORE 100 50 100 150 150 100 '00000148 GIRIDHAR RAJU K 1000 500 1000 1500 1500 1000 '00000153 SYAMALA DEVI T 1000 500 1000 1500 1500 1000 '00000154 SESHAGIRI RAO T 1500 750 1500 2250 2250 1500 '00000156 NALINI S 900 450 900 1350 1350 900 '00000157 JHANSI RANI P 500 250 500 750 750 500 '00000168 RADHAKRISHNA REDDY THAMMIREDDY 1000 500 1000 1500 1500 1000 '00000169 GUNUPATI AUDISESHA REDDY 1000 500 1000 1500 1500 1000 '00000176 NESAR AHMAD 1000 500 1000 1500 1500 1000 '00000182 LALITHA RAVI 500 250 500 750 750 500 '00000192 RAJESH KUMAR 100 50 100 150 150 100 '00000193 VARUN KUMAR 1300 650 1300 1950 1950 1300 1 THE WATERBASE LIMITED List of Shareholders whose Unclaimed Dividend and Shares Liable to be Transferred to IEPF / IEPFA Dividend Dividend Dividend Dividend Dividend Shares Liable to Folio / DP_Client ID Name of Shareholder (Interim - (Final - 2014- (Final - 2016- (Final - 2017- (Final - 2018- be Transferred 2014-15) 15) 17) 18) 19) to IEPFA Rs. -
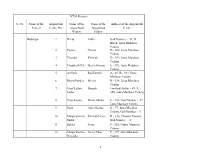
S. No. Name of the Project Anganwadi Centre No. Name of The
ICDS Projects S. No. Name of the Anganwadi Name of the Name of the Address of the Anganwadi Project Centre No. Anganwadi Anganwadi Centre Worker Helper Babarpur 1 Neetu Lalita Gali Number - 49, D Block, Janta Mazdoor Colony 2 Pavitra Chetna D - 362, Janta Mazdoor Colony 3 Virendri Vimlesh D - 282, Janta Mazdoor Colony 4 Chandresh Pal Geeta Sharma L - 392, Janta Mazdoor Colony 5 Archana Raj Kumari A - 49, B - 383, Janta Mazdoor Colony 6 Bharti Pandey Meena B - 334, Janta Mazdoor Colony 7 Vijay Laxmi Deepali Jamshed Anwar - 49 / L - Jaidev 350, Janta Mazdoor Colony 8 Vijay Laxmi Devki Aklota L - 132, Gali Number - 27, Janta Mazdoor Colony 9 Rajni Anju Sharma K - 97, Janta Mazdoor Colony, Gali Number - 5 10 Manju Sharma Vimlesh Deva K - 336, Chaman Panwali, Sushil Gali Number - 4 11 Babita Sonia F - 555, Nazta, Mazdoor Colony 12 Manju Sharma Geeta Vikas F - 179, Janta Mazdoor Devender Colony 1 13 Bharti Vandarna I - 30, Janta Mazdoor Maheswari Colony 14 Akshma Sharma Sunita Om I - 58, Block Khazoor Wali Gali, Janta Mazdoor Colony 15 Sangeeta Poonam Goyal A - 338, Idgah Road, Janta Mazdoor Colony 16 Jayshree Poonam Pawan J - 160, Janta Mazdoor Colony 17 Anjana Kaushik Shradha E - 49, B - 60, Janta Mazdoor Colony 18 Pooja Kaushik Sarvesh E - 49, D - 265, Janta Mazdoor Colony 19 Neetu Singh Rita Sharma E - 49, E - 11, Janta Mazdoor Colony 20 Konika Sharma Sunita Anil E - 49 / 128, Janta Mazdoor Colony 21 Monika Sharma Prem Lata D - 96, Gali Number - 3, Janta Mazdoor Colony 22 Rajeshwari Poonam Manoj W - 586, Gali Number - 3 / 8, Sudama Puri -

Supreme Court of India [ It Will Be Appreciated If The
SUPREME COURT OF INDIA [ IT WILL BE APPRECIATED IF THE LEARNED ADVOCATES ON RECORD DO NOT SEEK ADJOURNMENT IN THE MATTERS LISTED BEFORE ALL THE COURTS IN THE CAUSE LIST ] WEEKLY LIST No. 13 OF 2020 FROM : 05-08-2020 To 06-08-2020 COURT NO. : 4 HON'BLE MR. JUSTICE ROHINTON FALI NARIMAN HON'BLE MR. JUSTICE NAVIN SINHA HON'BLE MS. JUSTICE INDIRA BANERJEE NOTE:- [ ON 05.08.2020 ] REGULAR HEARING SNo. Case No. Petitioner / Respondent Petitioner/Respondent Advocate 1 C.A. No. 2742/2009 MONAPPA KOTTARI(D) BY LRS. V. N. RAGHUPATHY[P-1] IV-A Versus ANIL KUMAR MISHRA-I[R-1], ROMY DAVID PINTO AND ANR. CHACKO[R-2] 2 C.A. No. 5782/2008 INDUSTRIAL CABLES (I) LTD. MANJU JETLEY IV Versus COMMISSIONER OF INCOME B. V. BALARAM DAS, ANIL KATIYAR TAX,PATIALA and IA No.96571/2017-PERMISSION TO FILE ADDITIONAL DOCUMENTS Connected INDUSTRIAL CABLES (I) LTD. MANJU JETLEY C.A. No. 5784/2008 IV Versus COMMR.OF INCOME TAX,PATIALA B. V. BALARAM DAS, ANIL KATIYAR (PUNJAB) and FOR PERMISSION TO FILE ADDITIONAL DOCUMENTS ON IA 96568/2017 Connected INDUSTRIAL CABLES (INDIA) LTD. MANJU JETLEY C.A. No. 5785/2008 IV Versus COMMR.OF INCOME TAX,PATIALA B. V. BALARAM DAS, ANIL KATIYAR (PUNJAB) and FOR PERMISSION TO FILE ADDITIONAL DOCUMENTS ON IA 96579/2017 Connected INDUSTRIAL CABLES (INDIA) LIMITED MANJU JETLEY C.A. No. 7688/2009 IV Versus COMMR.OF INCOME TAX,PATIALA B. V. BALARAM DAS, ANIL KATIYAR and FOR PERMISSION TO FILE ADDITIONAL DOCUMENTS ON IA 96574/2017 GEETHA KOVILAN[P-1][PR], VIKAS 3 FERRODOUS ESTATES (PVT) LTD. -

List of Officers Who Attended Courses at NCRB
List of officers who attened courses at NCRB Sr.No State/Organisation Name Rank YEAR 2000 SQL & RDBMS (INGRES) From 03/04/2000 to 20/04/2000 1 Andhra Pradesh Shri P. GOPALAKRISHNAMURTHY SI 2 Andhra Pradesh Shri P. MURALI KRISHNA INSPECTOR 3 Assam Shri AMULYA KUMAR DEKA SI 4 Delhi Shri SANDEEP KUMAR ASI 5 Gujarat Shri KALPESH DHIRAJLAL BHATT PWSI 6 Gujarat Shri SHRIDHAR NATVARRAO THAKARE PWSI 7 Jammu & Kashmir Shri TAHIR AHMED SI 8 Jammu & Kashmir Shri VIJAY KUMAR SI 9 Maharashtra Shri ABHIMAN SARKAR HEAD CONSTABLE 10 Maharashtra Shri MODAK YASHWANT MOHANIRAJ INSPECTOR 11 Mizoram Shri C. LALCHHUANKIMA ASI 12 Mizoram Shri F. RAMNGHAKLIANA ASI 13 Mizoram Shri MS. LALNUNTHARI HMAR ASI 14 Mizoram Shri R. ROTLUANGA ASI 15 Punjab Shri GURDEV SINGH INSPECTOR 16 Punjab Shri SUKHCHAIN SINGH SI 17 Tamil Nadu Shri JERALD ALEXANDER SI 18 Tamil Nadu Shri S. CHARLES SI 19 Tamil Nadu Shri SMT. C. KALAVATHEY INSPECTOR 20 Uttar Pradesh Shri INDU BHUSHAN NAUTIYAL SI 21 Uttar Pradesh Shri OM PRAKASH ARYA INSPECTOR 22 West Bengal Shri PARTHA PRATIM GUHA ASI 23 West Bengal Shri PURNA CHANDRA DUTTA ASI PC OPERATION & OFFICE AUTOMATION From 01/05/2000 to 12/05/2000 1 Andhra Pradesh Shri LALSAHEB BANDANAPUDI DY.SP 2 Andhra Pradesh Shri V. RUDRA KUMAR DY.SP 3 Border Security Force Shri ASHOK ARJUN PATIL DY.COMDT. 4 Border Security Force Shri DANIEL ADHIKARI DY.COMDT. 5 Border Security Force Shri DR. VINAYA BHARATI CMO 6 CISF Shri JISHNU PRASANNA MUKHERJEE ASST.COMDT. 7 CISF Shri K.K. SHARMA ASST.COMDT. -

Allotment of Points : Ukg (2018-19)
ALLOTMENT OF POINTS : UKG (2018-19) PARENTS ARE REQUESTED TO CHECK THE POINTS ALLOTED TO THEIR WARDS AND IN CASE OF DISCREPANCY, INFORM THE SCHOOL BY 12 FEBRUARY 2018 BETWEEN 0900 HRS TO 1200 HRS POSITIVELY. PLEASE NOTE THAT NO REPRESENTATION WILL BE ENTERTAINED AFTER 12 FEBRUARY, 2018. PRINCIPAL MARKS ALLOTTED TO CHILDREN FOR ADMISSION TO UKG (2018-19) Points Points by by S.No. Regn. No. Student's Name DOB Father's Name Mother's Name Parents School Remarks 1 6001 SHUBH BELWAL 28-02-2012 RAJENDER SINGH BELWAL LAKSHMI 20 0 OVERAGE(6+) 2 6002 SHAURYA SUKHWANI 08-11-2013 SUNNY SUKHWANI NEHA SUKHWANI 25 25 3 6004 NAVYA GULATI 28-04-2013 VAIBHAV GULATI DEEPIKA GULATI 40 40 4 6005 S SANJANA 15-03-2014 K SHASHI KUMAR K KALPANA 55 55 5 6006 DIVYANSH DAS 07-05-2013 BANIBRATA DAS KABERI KAR 20 15 6 6007 DEVESH SINGH 02-09-2013 SUNIL SINGH MANJU SINGH 15 15 7 6008 MANISH 22-04-2013 SANDEEP OMWATI 25 25 8 6011 PRAGUN KUMAR 26-12-2013 BIJENDER KUMAR KUSUM LATA 10 0 ADDRESS PROOF DOESN'T MATCH 9 6013 SPARSH RAJ 17-09-2013 RAHUL RAJ ASMITA SINGH RAWAT 25 25 10 6014 AAROHI VERMA 23-09-2013 PUNIT KUMAR VERMA RUPAL VERMA 30 30 11 6017 AKSHITA 24-04-2013 DHARMENDER POONAM 35 35 12 6018 ANVI BANSAL 24-01-2013 ABHAY BANSAL MONIKA BANSAL 35 0 OVERAGE(5+) 13 6019 DIVYANSH SINGH 03-12-2013 RAVI KUMAR SINGH RICHA RATNAM 45 45 14 6020 MAHIN KHAN 17-11-2013 SHAHAB ALI KHAN UMAIRA KHAN 30 30 15 6021 AARADHYA PANDEY 11-10-2013 ABHISHEK GAUTAM PRATIBHA KUMARI 35 35 16 6022 ARADHYIA BAJPAI 01-02-2014 RAM KUMAR BAJPAI RAKHI BAJPAI 35 35 17 6023 SAMRAT BHARDWAJ 08-03-2013 -

COVID19 Prophylaxis and Its Final Report
HOMOEOPROPHYLAXIS AGAINST COVID19 As per guidelines of Ministry of AYUSH Government of India Dr. Rajneesh Kumar Sharma MD (Homoeopathy) Homoeo Cure & Research Institute Phone: NH-74, Dr. Rajneesh Marg, Moradabad +919897618594 Road, Kashipur (Uttarakhand) 244713 Email: INDIA [email protected] City, ST ZIP Code 1 1 Contents Courtesy 2 Introduction 3 Role of Homoeopathy in COVID19 pandemic 4 General preventive measures 4 Homoeoprophylaxis against COVID19 5 Followup Report after 40 days of Homoeoprophylaxis against COVID19 6 List of persons who have been prescribed or received the Ars alb 30CH as Homoeoprophylaxis against COVID19 12 www.cureme.org.in 2 Courtesy We are cordially thankful to respected Director Homoeopathy, Uttarakhand, Dr. Rajendra Singh, for his motivation, precious guidance and continuous support in this Herculian endeavor to support the world during this crucial time due to CORONA pandemic through the holistic system of healing i.e. Homoeopathy, by boosting up the immune system of the humanity with Homoeopathic prophylaxis with Ars alb 30 CH.tu www.cureme.org.in 3 Introduction Homoeo Cure & Research Institute has been founded by Dr. Rajneesh Kumar Sharma MD (Homoeopathy) in 1994 and providing health solutions to the suffering humanity with classical Homoeopathy all over the world. More than 25 countries in the world and all the states of India are seeking consultations from Dr. Rajneesh Kumar Sharma and getting very satisfactory results. Dr. Sharma is treating acute emergencies with Homoeopathic remedies in well-equipped ICU and NICU at his Sparsh Multispecialty Hospital, situated at Kashipur. In social field, he is actively participating with help of camps, surveys, publishing articles, presenting research papers in various seminars and various projects. -

MANALI PETROCHEMICALS LIMITED CIN : L24294TN1986PLC013087 Regd Off: 'SPIC HOUSE', 88, Mount Road, Guindy, Chennai- 600 032
MANALI PETROCHEMICALS LIMITED CIN : L24294TN1986PLC013087 Regd Off: 'SPIC HOUSE', 88, Mount Road, Guindy, Chennai- 600 032. Tele-Fax No.: 044-22351098 Email: [email protected], Website: www.manalipetro.com DETAILS OF SHARES TO BE TRANSFERRED TO INVESTOR EDUCATION & PROTECTION FUND ON WHICH NO DIVIDEND HAS BEEN CLAIMED FOR THE FY 2008-09 TO 2015-16 SL.NO FOLIO_DP_ID_CL_ID NAME OF THE SHAREHOLDER NO.OF.SHARES TOBE TRFD TO IEPF 1 A0000033 SITARAMAN G 450 2 A0000089 LAKSHMANAN CHELLADURAI 300 3 A0000093 MANI N V S 150 4 A0000101 KUNNATH NARAYANAN SUBRAMANIAN 300 5 A0000120 GOPAL THACHAT MURALIDHAR 300 6 A0000130 ROY FESTUS 150 7 A0000140 SATHYAMURTHY N 300 8 A0000142 MOHAN RAO V 150 9 A0000170 MURALIDHARAN M R 300 10 A0000171 CHANDRASEKAR V 150 11 A0000187 VISWANATH J 300 12 A0000191 JAGMOHAN SINGH BIST 300 13 A0000213 MURUGANANDAN RAMACHANDRAN 150 14 A0000219 SHANMUGAM E 600 15 A0000232 VENKATRAMAN N 150 16 A0000235 KHADER HUSSAINY S M 150 17 A0000325 PARAMJEET SINGH BINDRA 300 18 A0000332 SELVARAJU G 300 19 A0000334 RAJA VAIDYANATHAN R 300 20 A0000339 PONNUSWAMY SAMPANGIRAM 300 21 A0000356 GANESH MAHADHEVAN 150 22 A0000381 MEENAKSHI SUNDARAM K 150 23 A0000389 CHINNIAH A 150 24 A0000392 PERUMAL K 300 25 A0000423 CHANDRASEKARAN C 300 26 A0000450 RAMAMOORTHY NAIDU MADUPURI 150 27 A0000473 ZULFIKAR ALI SULTAN MOHAMMAD 300 28 A0000550 SRINIVASAN K 150 29 A0000556 KANAKAMUTHU A 300 30 A0000561 KODANDA PANI CHIVUKULA 300 31 A0000565 VARADHAN R 150 32 A0000566 KARTHIGEYAN S 150 33 A0000598 RAMASASTRULU TRIPIRNENI 150 34 A0000620 -

Year Book of the Indian National Science Academy
AL SCIEN ON C TI E Y A A N C A N D A E I M D Y N E I A R Year Book B of O The Indian National O Science Academy K 2019 2019 Volume I Angkor, Mob: 9910161199 Angkor, Fellows 2019 i The Year Book 2019 Volume–I S NAL CIEN IO CE T A A C N A N D A E I M D Y N I INDIAN NATIONAL SCIENCE ACADEMY New Delhi ii The Year Book 2019 © INDIAN NATIONAL SCIENCE ACADEMY ISSN 0073-6619 E-mail : esoffi [email protected], [email protected] Fax : +91-11-23231095, 23235648 EPABX : +91-11-23221931-23221950 (20 lines) Website : www.insaindia.res.in; www.insa.nic.in (for INSA Journals online) INSA Fellows App: Downloadable from Google Play store Vice-President (Publications/Informatics) Professor Gadadhar Misra, FNA Production Dr VK Arora Shruti Sethi Published by Professor Gadadhar Misra, Vice-President (Publications/Informatics) on behalf of Indian National Science Academy, Bahadur Shah Zafar Marg, New Delhi 110002 and printed at Angkor Publishers (P) Ltd., B-66, Sector 6, NOIDA-201301; Tel: 0120-4112238 (O); 9910161199, 9871456571 (M) Fellows 2019 iii CONTENTS Volume–I Page INTRODUCTION ....... v OBJECTIVES ....... vi CALENDAR ....... vii COUNCIL ....... ix PAST PRESIDENTS OF THE ACADEMY ....... xi RECENT PAST VICE-PRESIDENTS OF THE ACADEMY ....... xii SECRETARIAT ....... xiv THE FELLOWSHIP Fellows – 2019 ....... 1 Foreign Fellows – 2019 ....... 154 Pravasi Fellows – 2019 ....... 172 Fellows Elected (effective 1.1.2019) ....... 173 Foreign Fellows Elected (effective 1.1.2019) ....... 177 Fellowship – Sectional Committeewise ....... 178 Local Chapters and Conveners ...... -

23-AUG-2014 Investment Type Amount for Unclaimed and Unpaid Dividend
Company Name FLEX FOODS LIMITED CIN L15133UR1990PLC023970 Date of AGM(DD-MON-YYYY) 23-AUG-2014 Sum of unpaid and unclaimed dividend 1156576.00 Investment Type Amount for unclaimed and unpaid dividend Name Father/Husband Name Address Country State District Pin Folio No. of Amount Proposed date of Code Securities (in Rs.) transfer to IEPF A A SIDDIQUI IFTIKHAR AHMED VAIBHAV INVSTMENTS JAIN MANDI KHATAULI 251201 251201 INDIA Uttar Pradesh Muzaffarnagar 251201 0047465 1,000.00 08-OCT-2018 A CHANDRA KUMAR K ARDHANARY 102 P T RAJAN ROAD MADURAI TAMILNADU 625014 625014 INDIA Tamil Nadu Madurai 625014 0041420 200.00 08-OCT-2018 A K MUTHUSAMY GOUNDER KUMARASAMY GOUNDER C/O A MAGUDAPATHI D-1 PSTI QTRS BSK 2ND STAGE INDIA Karnataka Bangalore Urban 560070 0002458 200.00 08-OCT-2018 AGRIWLTURA BANGALORE 560070 560070 A K SINGH R P SINGH HOUSE NO 2278 CHUNA MANDI PAHAR GANJ NEW DELHI INDIA Delhi Central Delhi 110055 0035799 200.00 08-OCT-2018 110055 A K SRIDHARAN MARINE EINGINEER 8 R R FLATS 3-4 ANTHU STREET SANTHOME MADRAS INDIA Tamil Nadu Chennai 600004 0025318 200.00 08-OCT-2018 600004 600004 A MANICKAM NOT AVAILABLE 1690 16TH MAIN ROAD ANNA NAGAR MADRAS 600040 INDIA Tamil Nadu Chennai 600040 0025351 200.00 08-OCT-2018 600040 A PANDURANGA RAO NOT AVAILABLE C/O N NAGA RAJU ADVOCATE JAGANNADHAPURAM INDIA Andhra Pradesh Krishna 521001 0025220 200.00 08-OCT-2018 MACHILIPATNAM ANDHRA PRADESH 521001 A R R SELVARETHINAM RAJARETHINAM 4 VYABARIGAL STREET MAYLADUTHURAI 609003 609003 INDIA Tamil Nadu Thanjavur 609003 0025071 200.00 08-OCT-2018 A RAVINDRAN PILLAI -

Arun Kumar Sharma
LIVING LEGENDS IN INDIAN SCIENCE Arun Kumar Sharma Umesh C. Lavania Arun Kumar Sharma (AKS), a celebrated on a land donated to him by his father’s his maternal uncle, a lawyer. He grew up plant scientist of our country, is an inter- maternal uncle Mahamahopadhyay Gana- in a large joint family, loved by many of nationally acclaimed cytogeneticist and nath Sen. Construction was stopped due his grandma’s relatives. His maternal un- cytotaxonomist. He attained scientific to the untimely death of his father and cle took care of his education. He had his expertise researching throughout in India financial problems. Arun was then taken early education at Mitra Institution (1933– without going abroad for any formal to his maternal grandparents’ home by 39). Arun could not join the Presidency training. However, he has delivered lec- tures at various scientific venues through- out the world and has attracted scholars from far and wide. His dedication to duty and sense of detachment are strongly in- fluenced by his long association with the Ramakrishna Mission. Besides his own research activities on various domains of plant science, he has significantly con- tributed to the policy, planning and for- mulation of scientific and technical programmes for the growth of science in India in general. AKS has had exceptional courage and conviction at the young age of 33 to start an international journal of cytology and allied topics The Nucleus (1958). He au- thored (jointly with Archana Sharma) the classic reference book Chromosome AKS in his office in Calcutta University, 27 December 2011 (photograph: Y. -

CIN L15133UR1990PLC023970 Updated Upto Date of Last AGM 05-AUG-2019 Unpaid and Unclaimed Dividend for the Year 2014-15 Sum of Unpaid and Unclaimed Dividend 1575706.50
Company Name FLEX FOODS LIMITED CIN L15133UR1990PLC023970 Updated upto Date of Last AGM 05-AUG-2019 Unpaid and unclaimed dividend for the year 2014-15 Sum of unpaid and unclaimed dividend 1575706.50 Name Father/Husband Name Address Country State District Pin Folio No. of Amount Proposed date of Code Securities (in Rs.) transfer to IEPF A A SIDDIQUI IFTIKHAR AHMED INDIA UTTAR Muzaffarnagar 251201 0047465 1125.00 23-SEP-2022 PRADESH A K MUTHUSAMY GOUNDER KUMARASAMY GOUNDER INDIA KARNATAKA Bangalore Rural 560070 0002458 225.00 23-SEP-2022 AGRIWLTURA A K SETHI NOT AVAILABLE INDIA DELHI West Delhi 110064 0003886 225.00 23-SEP-2022 A K SINGH R P SINGH INDIA DELHI Central Delhi 110055 0035799 225.00 23-SEP-2022 A K SRIDHARAN MARINE EINGINEER INDIA TAMIL NADU Chennai 600004 0025318 225.00 23-SEP-2022 A P GUPTA L HAR PERSHAD INDIA DELHI North Delhi 110052 0048546 225.00 23-SEP-2022 A P GUPTA L HAR PERSHAD INDIA DELHI North Delhi 110052 0048545 225.00 23-SEP-2022 A P GUPTA L HAR PERSHAD INDIA DELHI North Delhi 110052 0048544 225.00 23-SEP-2022 A P GUPTA LATE HAR PERSHAD INDIA DELHI North Delhi 110052 0048543 225.00 23-SEP-2022 A PRAKASH CHAND NOT AVAILABLE INDIA DELHI New Delhi 110001 0025879 225.00 23-SEP-2022 A R R SELVARETHINAM RAJARETHINAM INDIA TAMIL NADU Thanjavur 609003 0025071 225.00 23-SEP-2022 A RAVINDRAN PILLAI ACHUTHAN PILLAI INDIA UTTAR Gautam Buddha 201301 0030802 225.00 23-SEP-2022 PRADESH Nagar A S MAINI KISHAN SINGH MAINI INDIA CHANDIGAR Chandigarh 160022 0003828 225.00 23-SEP-2022 H A S RAJENDRA A SATYANARAYANA INDIA KARNATAKA