Study of the Hydrolytic Stability of Fine-Grained Ceramics Based on Y2.5Nd0.5Al5o12 Oxide with a Garnet Structure Under Hydrothermal Conditions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Active Site Separation of Photocatalytic Steam Reforming of Methane Using Gas‐Phase Photoelectrochemical System
Electronic Supplementary Material (ESI) for Chemical Communications. This journal is © The Royal Society of Chemistry 2021 Active site separation of photocatalytic steam reforming of methane using Gas‐Phase Photoelectrochemical system Tomoki Kujirai,a Akira Yamaguchi*,a Takeshi Fujita,b Hideki Abe,c Masahiro Miyauchi*a a Department of Materials Science and Engineering, Tokyo Institute of Technology, 2-12-1 Ookayama, Meguro-ku, Tokyo 152-8552, Japan b Department of Environmental Science and Engineering, Kochi University of Technology, 185 Miyanokuchi, Tosayamada, Kami, Kochi 782-8502, Japan c National Institute for Materials Science, 1-1 Namiki, Tsukuba, Ibaraki 305-0044, Japan Table of Contents 1. Methods 2. GPEC system (Figure S1). 3. SRM using the GPEC system (Figure S2). 4. Cell fabrication (Figure S3). 5. ESR measurement (Figure S4). 6. The reactor for the SRM test with Pt/YSZ powder catalyst (Figure S5). 7. Measurement of the transmitted light of the cell (Figure S6). 8. Measurement of the surface temperature of the cell under UV light irradiation (Figure S7). 9. The XRD patterns of the Pt/YSZ powder catalyst and the YSZ pellet before screen printing (Figure S8). 10. The TEM image of the Pt/YSZ powder catalyst (Figure S9). 11. The UV-Vis absorption spectra of the YSZ powder and the Pt/YSZ powder catalyst, and the Tauc plot of the YSZ powder (Figure S10). 12. Thermodynamic limits of SRM calculated using NASA-CEA (Figure S11). 13. The spectrum of the Hg-Xe lamp with various cutoff filters (Figure S12). 14. The spectra of the transmitted light of the cell (Figure S13). -

Mizuho BK Custody and Proxy Board Lot Size List OCT 27, 2020 21LADY
Mizuho BK Custody and Proxy Board Lot Size List OCT 27, 2020 Board Lot Stock Name (in Alphabetical Order) ISIN Code QUICK Code Size 21LADY CO.,LTD. 100 JP3560550000 3346 3-D MATRIX,LTD. 100 JP3410730000 7777 4CS HOLDINGS CO.,LTD. 100 JP3163300001 3726 A DOT CO.,LTD 100 JP3160590000 7063 A-ONE SEIMITSU INC. 100 JP3160660001 6156 A.D.WORKS GROUP CO.,LTD. 100 JP3160560003 2982 A&A MATERIAL CORPORATION 100 JP3119800005 5391 A&D COMPANY,LIMITED 100 JP3160130005 7745 A&T CORPORATION 100 JP3160680009 6722 ABALANCE CORPORATION 100 JP3969530009 3856 ABC-MART,INC. 100 JP3152740001 2670 ABHOTEL CO.,LTD. 100 JP3160610006 6565 ABIST CO.,LTD. 100 JP3122480001 6087 ACCESS CO.,LTD. 100 JP3108060009 4813 ACCESS GROUP HOLDINGS CO.,LTD. 100 JP3108190004 7042 ACCRETE INC. 100 JP3108180005 4395 ACHILLES CORPORATION 100 JP3108000005 5142 ACMOS INC. 100 JP3108100003 6888 ACOM CO.,LTD. 100 JP3108600002 8572 ACRODEA,INC. 100 JP3108120001 3823 ACTCALL INC. 100 JP3108140009 6064 ACTIVIA PROPERTIES INC. 1 JP3047490002 3279 AD-SOL NISSIN CORPORATION 100 JP3122030004 3837 ADASTRIA CO.,LTD. 100 JP3856000009 2685 ADEKA CORPORATION 100 JP3114800000 4401 ADISH CO.,LTD. 100 JP3121500007 7093 ADJUVANT COSME JAPAN CO.,LTD. 100 JP3119620007 4929 ADTEC PLASMA TECHNOLOGY CO.,LTD. 100 JP3122010006 6668 ADVAN CO.,LTD. 100 JP3121950004 7463 ADVANCE CREATE CO.,LTD. 100 JP3122100005 8798 ADVANCE RESIDENCE INVESTMENT CORPORATION 1 JP3047160001 3269 ADVANCED MEDIA,INC. 100 JP3122150000 3773 ADVANEX INC. 100 JP3213400009 5998 ADVANTAGE RISK MANAGEMENT CO.,LTD. 100 JP3122410008 8769 ADVANTEST CORPORATION 100 JP3122400009 6857 ADVENTURE,INC. 100 JP3122380003 6030 ADWAYS INC. 100 JP3121970002 2489 AEON CO.,LTD. 100 JP3388200002 8267 AEON DELIGHT CO.,LTD. -

Custody Newsflash Oct 18Th, 2019
Custody Newsflash Oct 18th, 2019 List of Securities (ISIN, Record date and Board lot size) ======================================================================================== Category: Securities Information Summary: Below is the monthly updated list of Japanese stocks (including REIT and ETF) listed in Stock Exchanges in Japan with details (identification code, record date and board lot size) Impact: None Action: Just for information ======================================================================================== R/D(Fiscal Board Total Equities Securities ISIN Quick year end)* lot size Issued (in thou) Nikkei 300 Stock Index Listed Fund JP3013190008 0710 1000 1319 28,209 Daiwa ETF-TOPIX JP3027620008 0710 10 1305 2,795,715 TOPIX Exchange Traded Fund JP3027630007 0710 10 1306 6,008,162 Daiwa ETF-Nikkei 225 JP3027640006 0710 1 1320 123,092 Nikkei 225 Exchange Traded Fund JP3027650005 0708 1 1321 264,521 Nikko Exchange Traded Index Fund 225 JP3027660004 0708 10 1330 127,696 Nippon Building Fund Inc. JP3027670003 0630 1 8951 1,412 Japan Real Estate Investment Corporation JP3027680002 0331 18952 1,385 iShares Core Nikkei 225 ETF JP3027710007 0809 1 1329 26,886 Nikko Exchange Traded Index Fund TOPIX JP3039100007 0708 100 1308 2,735,329 Japan Retail Fund Investment Corporation JP3039710003 0228 18953 2,618 Daiwa ETF-TOPIX Core30 JP3040100004 0710 10 1310 1,116 Daiwa ETF-TOPIX Electric Appliances JP3040110003 0710 10 1610 1,023 Daiwa ETF-TOPIX Banks JP3040130001 0710 100 1612 4,673 TOPIX Core 30 Exchange Traded Fund JP3040140000 0715 10 1311 2,441 TOPIX Electric Appliances Exchange Traded Fund JP3040150009 0715 10 1613 1,141 TOPIX Banks Exchange Traded Fund JP3040170007 0715 100 1615 144,234 ORIX JREIT Inc. JP3040880001 0228 1 8954 2,760 Japan Prime Realty Investment Corporation JP3040890000 0630 18955 923 Premier Investment Corporation JP3041770003 0430 1 8956 1,316 TOKYU REIT, Inc. -

Federal Register/Vol. 65, No. 156/Friday, August 11, 2000/Notices
49264 Federal Register / Vol. 65, No. 156 / Friday, August 11, 2000 / Notices were filed for the purpose of extending TX; DDI, Tokyo, Japan; Ericsson, San National Cooperative Research and the Act's provisions limiting the Diego, CA; Fujitsu, Kawasaki, Japan; Production Act of 1993, 15 U.S.C. 4301 recovery of antitrust plaintiffs to actual Hewlett Packard, Grenoble Cedex 9, et seq. (``the Act''), Multi Protocol Label damages under specified circumstances. France; Hyundai Electronics, Ichon, Switching Forum (``MPLS Forum'') has Specifically, Universitat Karlsruhe, Kyounki-do, Republic of Korea; IBM filed written notifications Karlsruhe, Germany; BioSystems S.A., Corporation, Somers, Republic of Korea; simultaneously with the Attorney Barcelona, Spain; Ken Leiner Associates IP Mobile, Richardson, TX; LG General and the Federal Trade (KLA), Wheaton, MD; Trialog, Paris, Information & Communications, San Commission disclosing (1) the identities France; Universitat des Saarlandes, Diego, CA; Lucent Technologies, of the parties and (2) the nature and Saarbrucken, Germany; and Manickavel Naperville, IL; Malibu Networks, El objectives of the venture. The Subramani, Boyds, MD have been added Dorado Hills, CA; Marconi notifications were filed for the purpose as parties to this venture. Communications, Coventry, New of invoking the Act's provisions limiting No other changes have been made in Century Park, United Kingdom; the recovery of antitrust plaintiffs to either the membership or planned Microsoft Communications, Redmond, actual damages under specified activity of the group research project. WA; Mobile.com, Bellevue, WA; circumstances. Pursuant to Section 6(b) Membership in this group research Motorola, Arlington Heights, IL; of the Act, the identities of the parties project remains open, and J Consortium, NARUS, Inc., Palo Alto, CA; NEC, are Advanced Internet Laboratory, Inc. -

JASIS 2019 Exhibitors
JASIS 2019 Exhibitors Elemental Scientic, Inc. A Elementar Japan K.K. a priori Inc. ELGA LabWater A&D Company, Limited ELIONIX INC. ACTAC.CO.,LTD Emerging Technologies Corporation. Acumentech Co., Ltd. entex inc. AD Science Inc. Eppendorf Co., Ltd. Advanced Energy Japan K.K. ERECTA International Corporation Advantec Toyo Kaisha, Ltd. ESPEC CORP. ADVANTEST CORPORATION ESPEC MIC CORP. Agilent Technologies Japan,Ltd. ETRI AINEX CO., LTD. Excimer,inc AIRIX Corp. Airtech Corporation AIVS Corporation F Alpha M.O.S. Japan K.K. Filgen, Inc. AMETEK Co, Ltd FLON CHEMICAL INC. AMR, Inc. FLON INDUSTRY CO., LTD. analytica-Messe Muenchen ForDx, Inc. Analytik Jena Japan Co., Ltd. Forum for Innovative Regenerative Medicine(FIRM) ANRITSU METER CO., LTD. FUJIKIN Incorporated Anton Paar Japan K.K. Fujitsu Limited Aomori Prefecture Quantum Science Center FUKUSHIMA INDUSTRIES CORP. APRO Science Institute, Inc. FUSO Co., Ltd. ARAM CORPORATION FUTA-Q,Ltd. AS ONE CORPORATION ASAHI KOHSAN Co.,Ltd Asahi Lab Commerce, Inc. G ASAHI LIFE SCIENCE CO.,LTD GASTEC CORPORATION ASAHI RUBBER INC. GC INSTRUMENTS ASAHI TECHNEION CO., LTD. GE Healthcare Japan Corporation ASCH JAPAN CO.,LTD GERSTEL K.K. ASI-AURORA GL Sciences Inc. ASICON Tokyo Ltd. Glass Expansion Pty. Ltd. Association for structure characterization Global Facility Center,HOKKAIDO UNIVERSITY ASTECH CORPORATION GTR TEC CORPORATION ATAGO CO., LTD. GVS Japan K.K. B H BAS Inc. hagataya.co.ltd BD Consulting L.L.C. Hakuto Co., Ltd. BeatSensing co.,ltd. HAMAMATSU PHOTONICS K.K. Beckman Coulter K.K. Hamilton Company Japan K.K. BEIJING LINGGONG TECHNOLOGY CO.,LTD Hanaichi UltraStructure Research Institute BETHL Co.,Ltd. -

Calibration Service
INECCI002_1208 CALIBRATION SERVICE CHINO Corporation India Pvt. Ltd. has newly setup the State-of-the-art Calibration Laboratory at DAMAN works for calibration of Infrared Radiation Thermometer. The calibration laboratory is well equipped with sophisticated Standard Radiation Thermometer, Standard Resistance Thermometer, Digital Multi Meter, Digital Indicating Thermometer and Black Body Furnaces. These standard equipments have been calibrated in accordance with National Standard of NMIJ. (National Metrology Institute of Japan) CHINO India starts providing IR Calibration service for any model and make of IR Thermometer in the temperature range 50 to 1450ºC. Traceability System Outline National Standard National Institute of Advance Industrial Science and Technology Japan Electric Meters Inspection Corporation CHINO Standard CHINO Laboratory (Japan calibration Service System Accredited Calibration body) CHINO INDIA ▶Digital Indicating Thermometer ▶Standard Resistance Thermometer ▶Digital Multi Meter ▶Standard Radiation Thermometer CUSTOMER Radiation Thermometer IR Calibration Laboratory (CHINO India Daman Works) Calibration Equipments Standard radiation thermometer Model: IR-RST90H Standard resistance thermometer Model: NRHS1-0 Digital indicating thermometer Model: CAB-F201 Digital multi meter Model: 34970A Black body furnace Model: IR-R24 IR-R26 IR-R27 INECCI002_1208 CHINO IR Thermometers ►On-line Type IR-CA Series IR-SA Series IR-BA Series High Speed, -50 to 3500ºC Robust and High-accuracy, IP67 50ms fast response Compact design ►Fiber Type IR-FA Series Fiber optic type, 70 to 3000ºC Multi-functions ►Hand-held Type IR-AH Series IR-H Series IR-TA Series Light weight, Easy operation Upto3000ºC with View-finder Built-in Laser sight, Pocket size CHINO calibration equipments New KT-H500 Series IR-R24,26,27 Series R800/C800 KT-H301 Realized -100ºC, Various temperature ranges Standard T/C, RTD Small size Comparison Comparative calibration Black body furnace Calibration equipment Headquarters & Mumbai Sales Office: 417 & 418, 4th Floor, B-wing, Shree Nand-Dham, Plot No. -
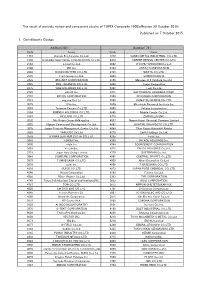
Published on 7 October 2015 1. Constituents Change the Result Of
The result of periodic review and component stocks of TOPIX Composite 1500(effective 30 October 2015) Published on 7 October 2015 1. Constituents Change Addition( 80 ) Deletion( 72 ) Code Issue Code Issue 1712 Daiseki Eco.Solution Co.,Ltd. 1972 SANKO METAL INDUSTRIAL CO.,LTD. 1930 HOKURIKU ELECTRICAL CONSTRUCTION CO.,LTD. 2410 CAREER DESIGN CENTER CO.,LTD. 2183 Linical Co.,Ltd. 2692 ITOCHU-SHOKUHIN Co.,Ltd. 2198 IKK Inc. 2733 ARATA CORPORATION 2266 ROKKO BUTTER CO.,LTD. 2735 WATTS CO.,LTD. 2372 I'rom Group Co.,Ltd. 3004 SHINYEI KAISHA 2428 WELLNET CORPORATION 3159 Maruzen CHI Holdings Co.,Ltd. 2445 SRG TAKAMIYA CO.,LTD. 3204 Toabo Corporation 2475 WDB HOLDINGS CO.,LTD. 3361 Toell Co.,Ltd. 2729 JALUX Inc. 3371 SOFTCREATE HOLDINGS CORP. 2767 FIELDS CORPORATION 3396 FELISSIMO CORPORATION 2931 euglena Co.,Ltd. 3580 KOMATSU SEIREN CO.,LTD. 3079 DVx Inc. 3636 Mitsubishi Research Institute,Inc. 3093 Treasure Factory Co.,LTD. 3639 Voltage Incorporation 3194 KIRINDO HOLDINGS CO.,LTD. 3669 Mobile Create Co.,Ltd. 3197 SKYLARK CO.,LTD 3770 ZAPPALLAS,INC. 3232 Mie Kotsu Group Holdings,Inc. 4007 Nippon Kasei Chemical Company Limited 3252 Nippon Commercial Development Co.,Ltd. 4097 KOATSU GAS KOGYO CO.,LTD. 3276 Japan Property Management Center Co.,Ltd. 4098 Titan Kogyo Kabushiki Kaisha 3385 YAKUODO.Co.,Ltd. 4275 Carlit Holdings Co.,Ltd. 3553 KYOWA LEATHER CLOTH CO.,LTD. 4295 Faith, Inc. 3649 FINDEX Inc. 4326 INTAGE HOLDINGS Inc. 3660 istyle Inc. 4344 SOURCENEXT CORPORATION 3681 V-cube,Inc. 4671 FALCO HOLDINGS Co.,Ltd. 3751 Japan Asia Group Limited 4779 SOFTBRAIN Co.,Ltd. 3844 COMTURE CORPORATION 4801 CENTRAL SPORTS Co.,LTD. -

Company Vendor ID (Decimal Format) (AVL) Ditest Fahrzeugdiagnose Gmbh 4621 @Pos.Com 3765 0XF8 Limited 10737 1MORE INC
Vendor ID Company (Decimal Format) (AVL) DiTEST Fahrzeugdiagnose GmbH 4621 @pos.com 3765 0XF8 Limited 10737 1MORE INC. 12048 360fly, Inc. 11161 3C TEK CORP. 9397 3D Imaging & Simulations Corp. (3DISC) 11190 3D Systems Corporation 10632 3DRUDDER 11770 3eYamaichi Electronics Co., Ltd. 8709 3M Cogent, Inc. 7717 3M Scott 8463 3T B.V. 11721 4iiii Innovations Inc. 10009 4Links Limited 10728 4MOD Technology 10244 64seconds, Inc. 12215 77 Elektronika Kft. 11175 89 North, Inc. 12070 Shenzhen 8Bitdo Tech Co., Ltd. 11720 90meter Solutions, Inc. 12086 A‐FOUR TECH CO., LTD. 2522 A‐One Co., Ltd. 10116 A‐Tec Subsystem, Inc. 2164 A‐VEKT K.K. 11459 A. Eberle GmbH & Co. KG 6910 a.tron3d GmbH 9965 A&T Corporation 11849 Aaronia AG 12146 abatec group AG 10371 ABB India Limited 11250 ABILITY ENTERPRISE CO., LTD. 5145 Abionic SA 12412 AbleNet Inc. 8262 Ableton AG 10626 ABOV Semiconductor Co., Ltd. 6697 Absolute USA 10972 AcBel Polytech Inc. 12335 Access Network Technology Limited 10568 ACCUCOMM, INC. 10219 Accumetrics Associates, Inc. 10392 Accusys, Inc. 5055 Ace Karaoke Corp. 8799 ACELLA 8758 Acer, Inc. 1282 Aces Electronics Co., Ltd. 7347 Aclima Inc. 10273 ACON, Advanced‐Connectek, Inc. 1314 Acoustic Arc Technology Holding Limited 12353 ACR Braendli & Voegeli AG 11152 Acromag Inc. 9855 Acroname Inc. 9471 Action Industries (M) SDN BHD 11715 Action Star Technology Co., Ltd. 2101 Actions Microelectronics Co., Ltd. 7649 Actions Semiconductor Co., Ltd. 4310 Active Mind Technology 10505 Qorvo, Inc 11744 Activision 5168 Acute Technology Inc. 10876 Adam Tech 5437 Adapt‐IP Company 10990 Adaptertek Technology Co., Ltd. 11329 ADATA Technology Co., Ltd. -

Constituent Changes TOPIX New Index Series (Effective 31 October 2016)
Constituent Changes TOPIX New Index Series (effective 31 October 2016) Published on 7 October 2016 1. Constituents Change (1) TOPIX Core30 Addition( 1 ) Deletion( 1 ) Code Issue Code Issue 6861 KEYENCE CORPORATION 8604 Nomura Holdings, Inc. (2) TOPIX Large70 Addition( 1 ) Deletion( 1 ) Code Issue Code Issue 8604 Nomura Holdings, Inc. 6861 KEYENCE CORPORATION (3) TOPIX Mid400 Addition( 7 ) Deletion( 7 ) Code Issue Code Issue 2201 Morinaga & Co.,Ltd. 1979 Taikisha Ltd. 3938 LINE Corporation 3608 TSI HOLDINGS CO.,LTD. 4043 Tokuyama Corporation 5021 COSMO ENERGY HOLDINGS COMPANY,LIMITED 4095 NIHON PARKERIZING CO.,LTD. 6740 Japan Display Inc. 4587 PeptiDream Inc. 6754 ANRITSU CORPORATION 7458 DAIICHIKOSHO CO.,LTD. 8368 The Hyakugo Bank,Ltd. 8585 Orient Corporation 8544 The Keiyo Bank,Ltd. (4) TOPIX Small Addition(14) Deletion( 7 ) Code Issue Code Issue 1979 Taikisha Ltd. 2201 Morinaga & Co.,Ltd. 3608 TSI HOLDINGS CO.,LTD. 3938 LINE Corporation 5021 COSMO ENERGY HOLDINGS COMPANY,LIMITED 4043 Tokuyama Corporation 6740 Japan Display Inc. 4095 NIHON PARKERIZING CO.,LTD. 6754 ANRITSU CORPORATION 4587 PeptiDream Inc. 8368 The Hyakugo Bank,Ltd. 7458 DAIICHIKOSHO CO.,LTD. 8544 The Keiyo Bank,Ltd. 8585 Orient Corporation * 3221 Yossix Co.,Ltd. * 3445 RS Technologies Co.,Ltd. * 3837 Ad-Sol Nissin Corporation * 3918 PCI Holdings,INC. * 6050 E-Guardian Inc. * 7527 SystemSoft Corporation * 9644 TANABE MANAGEMENT CONSULTING CO.,LTD. * This issue will be newly added to TOPIX new index series after the close of trading on October 28. 2. Number of constituents The Name of TOPIX New Index Current After (As of October 7) (Effective 30 October) TOPIX Core30 30 30 TOPIX Large70 70 70 TOPIX Mid400 400 400 TOPIX Small1 496 500 TOPIX Small2 974 977 Each code means constituents of following index. -

Ÿþj a P a N E S E B U S I N E S S E S T a B L I S H M E N T S I N I N D I A
Japanese Business Establishments in India November, 2011 Embassy of Japan in India About this list This list contains a total of 1422 establishments of Japanese business being operated in India. Please note that in case a company has business operations at multiple sites, each of them has a separate entry in this list (please also see "criteria" provided below). As of Oct 1st, 2011, 812 Japanese companies registered in India (in terms of the name of company registered in India) were observed to operate in India. Methodology Embassy of Japan in New Delhi and Consulate Generals of Japan in Mumbai, Chennai and Kolkata, and Consulate of Japan in Bangalore, have respectively collated information based on our research as well as information provided by the various regional Japanese Chambers of Commerce in India, Japan External Trade Organization (JETRO) in India, and others. Criteria for a "Japanese Business Establishment" in India In principle, any of the following is considered as a Japanese business establishment for the purpose of this list. However, some exceptional cases may also be included depending upon circumstances. 1. Liaison/Branch/Project Office of a Japanese company in India (as a foreign entity) 2. Indian subsidiary (wholly owned/joint venture) of a Japanese company, in the form of: (a)head office, (b)manufacturing unit, or (c)branch office with Japanese national(s) staying on regular basis 3. Business enterprise founded in India by Japanese national(s) State wise Japanese Business Establishments in India Chandigarh(1) Uttarakhand(6) -

USB/RS-422/485 Conversion Adapter Connection Guide
USB/RS-422/485 Conversion Adapter Connection Guide 1 Supported Drivers ............................................................................................................ 3 2 Cable Diagrams ...............................................................................................................5 1 USB/RS-422/485 Conversion Adapter Connection Guide Introduction This manual describes how to use a Pro-face’s USB/RS-422/485 Conversion Adapter (PFXZCBCBCVUSR41) to connect a Display and External Device. Please refer to the USB/RS-422/485 Conversion Adapter installation guide for details. GP-Pro EX Device/PLC Connection Manual 2 USB/RS-422/485 Conversion Adapter Connection Guide 1 Supported Drivers You can connect the following External Devices using the USB/RS-422/485 Conversion Adapter. Manufacturer Driver Name CHINO CORPORATION Temp. Controller MODBUS SIO Digital Electronics General SIO Corporation Memory Link Fatek Automation FB Series SIO Corporation Inverter SIO MICREX-F Series SIO Fuji Electric Co., Ltd MICREX-SX Series SIO Temp. Controller MODBUS SIO H Series SIO Hitachi Industrial Equipment Systems Co., Inverter ASCII SIO Ltd. Inverter MODBUS RTU Hyundai Heavy Industries Hi5 Robot IAI Corporation ROBO CYLINDER MODBUS SIO JTEKT Corporation TOYOPUC CMP-LINK SIO KEYENCE Corporation KV-700/1000/3000/5000 CPU Direct KOYO ELECTRONICS KOSTAC/DL Series CCM SIO INDUSTRIES GLOFA Series Cnet LS Industrial Systems MASTER-K Series Cnet Co.,Ltd. XGT/XGB Series Cnet A Series Computer Link FREQROL Inverter Mitsubishi Electric FX Series Computer Link Corporation FX Series CPU Direct Q/QnA Serial Communication Modbus-IDA General MODBUS SIO Master C/CV Series HOST Link Omron Corporation CS/CJ Series HOST Link Temp. Controller CompoWay/F Panasonic Electric Works FP Series Computer Link SIO SUNX Temp. Controller MODBUS SIO RKC Instrument Inc. -

Information Square Ulvac
ULVAC INFORMATION SQUARE Agreed to the Transfer of Iwatani Gas’s Renamed to ADVANCE RIKO with the Cryogenic Equipment Business to UCI Transfer of Shares to CHINO — ULVAC CRYOGENICS INCORPORATED — ULVAC, Inc. In May 2014, ULVAC CRYOGENICS In November 2014, a decision was made to INCORPORATED (hereinafter, UCI) took over transfer all shares of ULVAC-RIKO, Inc., a con- the manufacturing and selling of cryogenic equip- solidated subsidiary of ULVAC, Inc. to CHINO ment from the Cryogenic Equipment Department Corporation. On December 25, 2014, ULVAC- of the Engineering Division at Iwatani Industrial RIKO, Inc. left the ULVAC Group and made a Gases Corporation (hereinafter, Iwatani Gas) and new start under the name of ADVANCE RIKO, started manufacturing and selling the equipment. Inc. as part of the CHINO Group. UCI is a manufacturer specializing in Since its founding under the name of cryopumps used for vacuum devices and equip- SHINKU-RIKO Inc. in 1962, ULVAC-RIKO, ment—such as chip making equipment, flat panel Inc. has developed its business as a manufacturer display production equipment and optical film specializing in infrared heaters, thermal analysis production equipment—and has been supplying and thermal property measuring instruments and many cryopumps in Japan and Asia for many Liquid nitrogen server other types of thermal analysis equipment. years. In 2009, UCI began manufacturing and The transferee, CHINO Corporation, is a selling cryogenic freezers used in the supercon- manufacturer specializing in temperature and ductor, medical equipment, measuring instru- Various cryostats other measuring devices and controls, and pro- ments and laboratory cryostats sectors. vides integrated solutions—from measuring tem- As a major manufacturer of cryogenic equip- This has generated synergy that has led to posi- perature to regulating temperature—as its main ment (mainly cryostats and cryogenic freezers), tive technical innovations; and it facilitates entry business.