Appendix a Species Assessments and Conservation Measures
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Endangered Species
FEATURE: ENDANGERED SPECIES Conservation Status of Imperiled North American Freshwater and Diadromous Fishes ABSTRACT: This is the third compilation of imperiled (i.e., endangered, threatened, vulnerable) plus extinct freshwater and diadromous fishes of North America prepared by the American Fisheries Society’s Endangered Species Committee. Since the last revision in 1989, imperilment of inland fishes has increased substantially. This list includes 700 extant taxa representing 133 genera and 36 families, a 92% increase over the 364 listed in 1989. The increase reflects the addition of distinct populations, previously non-imperiled fishes, and recently described or discovered taxa. Approximately 39% of described fish species of the continent are imperiled. There are 230 vulnerable, 190 threatened, and 280 endangered extant taxa, and 61 taxa presumed extinct or extirpated from nature. Of those that were imperiled in 1989, most (89%) are the same or worse in conservation status; only 6% have improved in status, and 5% were delisted for various reasons. Habitat degradation and nonindigenous species are the main threats to at-risk fishes, many of which are restricted to small ranges. Documenting the diversity and status of rare fishes is a critical step in identifying and implementing appropriate actions necessary for their protection and management. Howard L. Jelks, Frank McCormick, Stephen J. Walsh, Joseph S. Nelson, Noel M. Burkhead, Steven P. Platania, Salvador Contreras-Balderas, Brady A. Porter, Edmundo Díaz-Pardo, Claude B. Renaud, Dean A. Hendrickson, Juan Jacobo Schmitter-Soto, John Lyons, Eric B. Taylor, and Nicholas E. Mandrak, Melvin L. Warren, Jr. Jelks, Walsh, and Burkhead are research McCormick is a biologist with the biologists with the U.S. -

Lamprey, Hagfish
Agnatha - Lamprey, Kingdom: Animalia Phylum: Chordata Super Class: Agnatha Hagfish Agnatha are jawless fish. Lampreys and hagfish are in this class. Members of the agnatha class are probably the earliest vertebrates. Scientists have found fossils of agnathan species from the late Cambrian Period that occurred 500 million years ago. Members of this class of fish don't have paired fins or a stomach. Adults and larvae have a notochord. A notochord is a flexible rod-like cord of cells that provides the main support for the body of an organism during its embryonic stage. A notochord is found in all chordates. Most agnathans have a skeleton made of cartilage and seven or more paired gill pockets. They have a light sensitive pineal eye. A pineal eye is a third eye in front of the pineal gland. Fertilization of eggs takes place outside the body. The lamprey looks like an eel, but it has a jawless sucking mouth that it attaches to a fish. It is a parasite and sucks tissue and fluids out of the fish it is attached to. The lamprey's mouth has a ring of cartilage that supports it and rows of horny teeth that it uses to latch on to a fish. Lampreys are found in temperate rivers and coastal seas and can range in size from 5 to 40 inches. Lampreys begin their lives as freshwater larvae. In the larval stage, lamprey usually are found on muddy river and lake bottoms where they filter feed on microorganisms. The larval stage can last as long as seven years! At the end of the larval state, the lamprey changes into an eel- like creature that swims and usually attaches itself to a fish. -

Fine Structure of the Retina of Black Bass, Micropterus Salmoides (Centrarchidae, Teleostei)
Histol Histopathol (1999) 14: 1053-1065 Histology and 001: 10.14670/HH-14.1053 Histopathology http://www.hh.um.es From Cell Biology to Tissue Engineering Fine structure of the retina of black bass, Micropterus salmoides (Centrarchidae, Teleostei) M. Garcia and J. de Juan Department of Biotechnology, University of Alicante, Alicante, Spain Summary. The structure of light- and dark-adapted 1968), 4) regular cone mosaics (Wagner, 1978), 5) the retina of the black bass, Micropterus salmoides has been existence of a well developed retinal tapetum in studied by light and electron microscopy. This retina nocturnal and bottom-living species (Wagner and Ali, lacks blood vessels at all levels. The optic fiber layer is 1978), 6) presence of foveae or areas with an increase in divided into fascicles by the processes of Muller cells photoreceptors and other neurons (Wagner, 1990), and 7) and the ganglion cell layer is represented by a single row a marked synaptic plasticity as is demonstrated by the of voluminous cells. The inner nuclear layer consists of formation of spinules (Wagner, 1980) during light two layers of horizontal cells and bipolar, amacrine and adaptation and their disappearance during dark interplexiform cells. In the outer plexiform layer we adaptation, and others. In summary, these features observed the synaptic terminals of photoreceptor celis, demonstrate the importance of vision in the mode of life rod spherules and cone pedicles and terminal processes and survival of the species. of bipolar and horizontal cells. The spherules have a Micropterus salmoides, known as black bass, is a single synaptic ribbon and the pedicles possess multiple member of the family Centrarchidae (Order Perciformes) synaptic ribbons. -

XIV. Appendices
Appendix 1, Page 1 XIV. Appendices Appendix 1. Vertebrate Species of Alaska1 * Threatened/Endangered Fishes Scientific Name Common Name Eptatretus deani black hagfish Lampetra tridentata Pacific lamprey Lampetra camtschatica Arctic lamprey Lampetra alaskense Alaskan brook lamprey Lampetra ayresii river lamprey Lampetra richardsoni western brook lamprey Hydrolagus colliei spotted ratfish Prionace glauca blue shark Apristurus brunneus brown cat shark Lamna ditropis salmon shark Carcharodon carcharias white shark Cetorhinus maximus basking shark Hexanchus griseus bluntnose sixgill shark Somniosus pacificus Pacific sleeper shark Squalus acanthias spiny dogfish Raja binoculata big skate Raja rhina longnose skate Bathyraja parmifera Alaska skate Bathyraja aleutica Aleutian skate Bathyraja interrupta sandpaper skate Bathyraja lindbergi Commander skate Bathyraja abyssicola deepsea skate Bathyraja maculata whiteblotched skate Bathyraja minispinosa whitebrow skate Bathyraja trachura roughtail skate Bathyraja taranetzi mud skate Bathyraja violacea Okhotsk skate Acipenser medirostris green sturgeon Acipenser transmontanus white sturgeon Polyacanthonotus challengeri longnose tapirfish Synaphobranchus affinis slope cutthroat eel Histiobranchus bathybius deepwater cutthroat eel Avocettina infans blackline snipe eel Nemichthys scolopaceus slender snipe eel Alosa sapidissima American shad Clupea pallasii Pacific herring 1 This appendix lists the vertebrate species of Alaska, but it does not include subspecies, even though some of those are featured in the CWCS. -

Winter Biology of Centrarchid Fishes C
Chapter 9 Winter biology of centrarchid fishes C. D. Suski and M. S. Ridgway 9.1 Introduction Temperate latitudes experience a predictable annual cycle of alternating warm and cold periods that can result in below freezing conditions, ice cover, and alterations to aquatic habitats that persist for a substantial portion of a year. Winter represents a very interesting and challenging time of the year that exerts a strong selective pressure on individual survival, community structure, and year class strength for centrarchid fishes. Despite the impact of this time on both individuals and populations, we are only beginning to comprehend how this period of the year can influence centrarchid fishes. The purpose of this chapter is to summarize the current literature that defines the ecological, behavioral, and physio- logical alterations experienced by centrarchid fishes both prior to and during winter. Because of the paucity of information on winter biology of centrarchid fishes, this chapter has been written in a general format whereby studies of different centrarchid fishes have been pooled to identify trends that exist across the entire family. Where appropriate, exceptions to these general trends have been noted. A general over-arching question does emerge from work to date despite the lack of broad research coverage in many areas of centrarchid winter biology: What physiological and ecological changes occur to ensure survival prior to and during a period of reduced energy intake? 9.2 Definition of “winter” We define “winter” as the period of the year between the autumnal equinox and prior to the onset of spawning in centrarchid fishes. -

Pennington Creek Fish
FAMILY: CENTRARCHIDAE (sunfishes) FAMILY: CYPRINIDAE (minnows) Bluegill Orangespotted Sunfish Smallmouth Bass Bigeye Shiner Lepomis macrochirus Lepomis humilis Micropterus dolomieu Notropis boops Characteristics: deep-bodied, small mouth, Characteristics: small with orange spots Characteristics: large mouth, vertical dark Characteristics: large eye relative to black spot posterior dorsal rays on side, long white-edged opercular flap bars are sometimes present on olive- body size, large mouth with a small bronze colored sides of the fish, juveniles head, dark lateral stripe extends from have an orange and black band on the cau- the lips through the eye to the end of dal fin the caudal peduncle Green Sunfish Redear Sunfish Largemouth Bass Blacktail Shiner Lepomis cyanellus Lepomis microlophus Micropterus salmoides Cyprinella venusta Characteristics: elongated body, large Characteristics: large, short opercular flap Characteristics: large mouth, upper jaw Characteristics: prominent black spot at mouth, black spot posterior dorsal & anal with a bright red crescent marking extends past the eye, dark midlateral the base of the caudal fin, large stripe from snout to base of the caudal fin diamond shaped scales outlined in black, breeding males develop yellow fins Longear Sunfish White & Black Crappie Spotted Bass Bluntnose Minnow Lepomis megalotis Pomoxis annularis, Pomoxis nigromacula- Micropterus punctulatus Pimephales notatus Characteristics: small, deep-bodied, long tus Characteristics: resembles the largemouth Characteristics: blunt, rounded -

Bill Beamish's Contributions to Lamprey Research and Recent Advances in the Field
Guelph Ichthyology Reviews, vol. 7 (2006) 1 Bill Beamish’s Contributions to Lamprey Research and Recent Advances in the Field This paper is based on an oral presentation given at a symposium honouring Bill Beamish and his contributions to fisheries science at the Canadian Conference for Fisheries Research, in Windsor, Ontario on January 7, 2005 Margaret F. Docker Great Lakes Institute for Environmental Research, University of Windsor, Windsor, ON, N9B 3P4, Canada Current Address: Department of Zoology, University of Manitoba, Winnipeg, MB, R3T 2N2, Canada (e-mail: [email protected]) Key Words: review, sex determination, statoliths, pheromones, reproductive endocrinology, phylogeny Guelph Ichthyology Reviews, vol. 7 (2006) 2 Synopsis Since his first lamprey paper in 1972, Bill Beamish has published more than 50 papers on numerous aspects of lamprey biology, reporting on several native lamprey species as well as the Great Lakes sea lamprey. Bill and his colleagues have contributed to our knowledge of the basic biology of larval lampreys (e.g., abundance, habitat, feeding, growth, and gonadogenesis), helped refine techniques to determine age in larvae (using statoliths, structures analogous to the teleost otolith), and studied the process of metamorphosis and the feeding and bioenergetics of juvenile (parasitic) lampreys. Current research continues to build on Bill’s contributions, and also makes many advances in novel directions. This exciting current research includes: the use of high-resolution ultrasound to study gonadogenesis and evaluate sex ratio in live larval lampreys; the elucidation of some of the exogenous and endogenous triggers of metamorphosis; examination of the neuroendocrine control of reproduction and the role of unconventional sex steroids in lampreys; the discovery of migratory and sex pheromones and their potential use in sea lamprey control; the use of molecular markers to study lamprey mating systems and phylogeny; and the renewed interest in the conservation of native lampreys. -

Assessment of the Bird Or Animal Deformities Or Reproductive Problems Beneficial Use Impairment in Michigan’S Great Lakes Areas of Concern 2020
MI/EGLE/WRD-20/002 Assessment of the Bird or Animal Deformities or Reproductive Problems Beneficial Use Impairment in Michigan’s Great Lakes Areas of Concern 2020 Prepared by: Dennis Bush, Toxicologist Brandon Armstrong, Toxicologist Sarah Bowman, Toxicologist Joseph Bohr, Aquatic Biologist Surface Water Assessment Section Water Resources Division January 2020 ACKNOWLEDGEMENTS We would like to thank Dr. Lisa Williams and Mandy Annis of the United States Fish and Wildlife Service for providing Great Lakes Restoration Initiative funds for this project. Additional funding was also provided by the Clean Michigan Initiative. We would also like to thank University of Maryland staff (Dr. William Bowerman, Dr. Meredith Bohannon, and Hannah Evans) for providing the bald eagle and herring gull data and David Best for providing information regarding specific eagle territories. We would also like to thank Dr. Steven Bursian for facilitating the analysis of the mink jaws. We would like to thank Dr. Keith Grasman for providing information and data on herring gulls, Caspian terns, and black-crowned night herons. Lastly, we would like to thank Mr. Joe Medema for providing the mink and muskrats from the Kalamazoo River. Cover photos: Common tern - Katherine Whittemore, United States Fish and Wildlife Service; Bald eagle - Dr. William Bowerman, University of Maryland; and Mink - Don Breneman, Great Lakes National Program Office. Table of Contents_Toc26367018 EXECUTIVE SUMMARY .......................................................................................................... -

Elassoma Alabamae, Anew Species of Pygmy Sunfish Endemic to the Tennessee River Drainage of Alabama (Teleostei: Elassomatidae)
Number 16 June 15, 1993 Elassoma alabamae, aNew Species of Pygmy Sunfish Endemic to the Tennessee River Drainage of Alabama (Teleostei: Elassomatidae) ANew Species of Percina (Odontopholis) from Kentucky and Tennessee with Comparisons to Percina cymatotaenia (Teleostei: Percidae) Systematics of the Etheostoma jordani Species Group (Teleostei: Percidae), with Descriptions of Three New Species BULLETIN ALABAMA MUSEUM OF NATURAL HISTORY The scientific publication of the Alabama Museum of Natural History. Richard L. Mayden, Editor,john C. Hall, Managing Editor. BULLETIN ALABAMA MUSEUM OF NATURAL HISTORYis published by the Alabama Museum of Natural History, a unit of The University of Alabama. The BULLETIN succeeds its predecessor, the MUSEUM PAPERS, which was termi nated in 1961 upon the transfer of the Museum to the University from its parent organization, the Geological Survey of Alabama. The BULLETIN is devoted primarily to scholarship and research concerning the natural history of Alabama and the Midsouth. It appears irregularly in consecutively numbered issues. Communication concerning manuscripts, style, and editorial policy should be addressed to: Editor, BULLETIN ALABAMA MUSEUM OF NATURAL HIS TORY, The University of Alabama, Box 870340, Tuscaloosa, AL 35487-0340; Telephone (205) 348-7550. Prospective authors should examine the Notice to Authors inside the back cover. Orders and requests for general information should be addressed to Managing Editor, BULLETIN ALABAMA MUSEUM OF NATURAL HISTORY, at the above address. Numbers may be purchased individually; standing orders are accepted. Remittances should accompany orders for individual numbers and be payable to The University of Alabama. The BULLETIN will invoice standing orders. Library exchanges may be handled through: Exchange Librarian, The University of Alabama, Box 870266, Tuscaloosa, AL 35487-0340. -
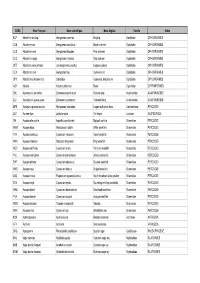
Nom Français
CODE Nom Français Nom scientifique Nom Anglais Famille Ordre KCP Abadèche du Cap Genypterus capensis Kingklip Ophidiidae OPHIDIIFORMES CUB Abadèche noir Genypterus maculatus Black cusk-eel Ophidiidae OPHIDIIFORMES CUS Abadèche rosé Genypterus blacodes Pink cusk-eel Ophidiidae OPHIDIIFORMES CUC Abadèche rouge Genypterus chilensis Red cusk-eel Ophidiidae OPHIDIIFORMES OFZ Abadèche sans jambes Lamprogrammus exutus Legless cuskeel Ophidiidae OPHIDIIFORMES CEX Abadèches nca Genypterus spp Cusk-eels nei Ophidiidae OPHIDIIFORMES OPH Abadèches, brotules nca Ophidiidae Cusk-eels, brotulas nei Ophidiidae OPHIDIIFORMES ALR Ablette Alburnus alburnus Bleak Cyprinidae CYPRINIFORMES ZML Acanthure à pierreries Zebrasoma gemmatum Spotted tang Acanthuridae ACANTHUROIDEI ZLV Acanthure à queue jaune Zebrasoma xanthurum Yellowtail tang Acanthuridae ACANTHUROIDEI MPS Achigan à grande bouche Micropterus salmoides Largemouth black bass Centrarchidae PERCOIDEI LQT Acmée râpe Lottia limatula File limpet Lottiidae GASTROPODA ISA Acoupa aile-courte Isopisthus parvipinnis Bigtooth corvina Sciaenidae PERCOIDEI WEW Acoupa blanc Atractoscion nobilis White weakfish Sciaenidae PERCOIDEI YNV Acoupa cambucu Cynoscion virescens Green weakfish Sciaenidae PERCOIDEI WKK Acoupa chasseur Macrodon ancylodon King weakfish Sciaenidae PERCOIDEI WEP Acoupa du Pérou Cynoscion analis Peruvian weakfish Sciaenidae PERCOIDEI YNJ Acoupa mongolare Cynoscion jamaicensis Jamaica weakfish Sciaenidae PERCOIDEI SWF Acoupa pintade Cynoscion nebulosus Spotted weakfish Sciaenidae PERCOIDEI WKS Acoupa -

Conservation Status of Imperiled North American Freshwater And
FEATURE: ENDANGERED SPECIES Conservation Status of Imperiled North American Freshwater and Diadromous Fishes ABSTRACT: This is the third compilation of imperiled (i.e., endangered, threatened, vulnerable) plus extinct freshwater and diadromous fishes of North America prepared by the American Fisheries Society’s Endangered Species Committee. Since the last revision in 1989, imperilment of inland fishes has increased substantially. This list includes 700 extant taxa representing 133 genera and 36 families, a 92% increase over the 364 listed in 1989. The increase reflects the addition of distinct populations, previously non-imperiled fishes, and recently described or discovered taxa. Approximately 39% of described fish species of the continent are imperiled. There are 230 vulnerable, 190 threatened, and 280 endangered extant taxa, and 61 taxa presumed extinct or extirpated from nature. Of those that were imperiled in 1989, most (89%) are the same or worse in conservation status; only 6% have improved in status, and 5% were delisted for various reasons. Habitat degradation and nonindigenous species are the main threats to at-risk fishes, many of which are restricted to small ranges. Documenting the diversity and status of rare fishes is a critical step in identifying and implementing appropriate actions necessary for their protection and management. Howard L. Jelks, Frank McCormick, Stephen J. Walsh, Joseph S. Nelson, Noel M. Burkhead, Steven P. Platania, Salvador Contreras-Balderas, Brady A. Porter, Edmundo Díaz-Pardo, Claude B. Renaud, Dean A. Hendrickson, Juan Jacobo Schmitter-Soto, John Lyons, Eric B. Taylor, and Nicholas E. Mandrak, Melvin L. Warren, Jr. Jelks, Walsh, and Burkhead are research McCormick is a biologist with the biologists with the U.S. -

Elassoma Zonatum, E. Okefenokee,Ande. Evergladei
Fall (Oct.) American Currents 8 Elassoma zonatum, E. okefenokee, and E. evergladei: Habitats and Comparative Observations By Jörg Bohlen and Arne Nolte Translated from the German (DATZ 1993) by Rudolf 60 sq m pool near the mouth of the Pascagoula River in G. Arndt, Professor Emeritus of Marine Science, The Mississippi. This pool had low water at the time of Richard Stockton College of New Jersey, Pomona, collection (late May 1991) and is directly connected New Jersey, USA with assistance from Gerald Pottern with a cypress swamp, which apparently caused the and used with permission of the authors. tea-brown color of the otherwise clear water. Near the shore was an approximately 1.5 m The species in the genus Elassoma include wide thicket of various submerged plants, twigs and fishes with a maximum length of 4.5 cm, and which leaves. We collected mostly juvenile E. zonatum (SL < occur only in the southeastern USA. All species have a 20 mm) only in this belt of vegetation, in which we also well-developed sexual dimorphism, in which the males collected Lepomis macrochirus, Centrarchus at the time of courtship have iridescent blue markings macropterus, Gambusia affinis, and Fundulus on a weak black background color. The females always chrysotus. Outside of the plant thicket we caught a have a camouflaging coloration of beiges and browns. water turtle (Pseudemys sp.) and various water snakes. Besides the brilliant colors of the males and the small Juvenile Elassoma zonatum occurred in sizes of these species, it is primarily the ”fluttering” abundance (20 to 30 specimens collected in 20 minutes), movements during courtship that makes these fishes so that each pull of a large, sturdy dip-net caught up to such appealing and interesting aquarium fishes.