Transcriptomic Signatures of Experimental Alkaloid Consumption in a Poison Frog
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Flyer Seniorenzentrum
AGAPLESION AGAPLESION BETHESDA SENIORENZENTRUM BETHESDA SENIORENZENTRUM UNNA UNNA ANFahrt Das AGAPLESION BETHESDA SENIORENZENTRUM UNNA liegt am östlichen Rand des Stadtteils Königsborn, eingebettet in eine idyllische Stadtrandlage, nahe der Ausfallstraße von Unna nach Hamm. Von hier aus erreichen Sie schnell den angrenzenden Kurpark und die historisch gewachsene Innenstadt mit ihrer schö- nen Fußgängerzone und vielen Geschäften. ZUHAUSE IN CHRISTLICHER Eine Bushaltestelle ist in 200 Metern leicht erreichbar, der S- Bahnhof Unna-Königsborn in etwa 400 Metern. GEBORGENHEIT Ihr Partner Ihre Ansprechpartner Mit öffentlichen Verkehrsmitteln: • ab Bahnhof Unna mit dem Regio-Bus R53 Mit Liebe zum Leben Sicherlich haben Sie Fragen oder möchten sich AGAPLESION wurde 2002 als gemeinnützige Richtung Heeren Denkmal, selbst ein Bild machen. Der Leiter der Einrichtung, Aktiengesellschaft in Frankfurt am Main gegründet Haltestelle Königsborn Waalwijker Straße, Stefan Sikora, und sein Team stehen Ihnen jederzeit für mit dem Ziel, christliche und soziale Einrichtungen etwa 400 Meter Fußweg ein Gespräch zur Verfügung und beraten Sie zu auch in einer anspruchsvollen Wirtschafts- und allen Fragen, Anliegen und notwendigen Formalitäten. Wettbewerbssituation zu stärken. AGAPLESION www.bethesda-unna.de Als christlicher Konzern im Sozial- und Gesund- BETHESDA SENIORENZENTRUM UNNA Weitere Informationen oder Termine für eine heitswesen behandelt und betreut AGAPLESION Hammer Str. 102K • 59425 Unna / Königsborn Hausführung geben wir Ihnen gerne unter Menschen in -

Radiox – Radiology – Nuclear Medicine – Radiotherapy Soest, Germany
RADIOLOGY WORKFLOW SOLUTIONS Growing “Big and Strong” together IT Development driven by Needs and used with Pleasure The imaging center for radiology-nuclear-medicine-radiotherapy Soest, radiox for short, has one of the most modern facilities for radiotherapy in Germany. The imaging center is linked to the hospital Maria in Soest since their foundation in 1998. 21 doctors and 150 employees in Soest and four further locations attend both out- patients and inpatients in close cooperation with more medical utilities in the region. Communication between the facilities is supported by the RADIOLOGY INFORMA- TION SYSTEM (RIS) from the supplier medavis. It controls the complete workflow, communicates internally with the modalities and externally with the hospital infor- mation system (HIS). This simplifies the work for the medical employees and for the patient it means to have a holistic treatment, fast diagnostical decision-making and optimal therapeutical strategies without waiting time. Digitalisation ensures growth everyone enlisted in the treatment must work together. Soon after the foundation of radiox in 1998 it was clear Collaboration includes talking to each other and pro- in the doctors’ mind that they have to grow wider and viding information, and in doing so we already have establish new sites in the western Sauerland in order reached the backbone of our daily work: RIS” states Mr. to treat patients professionally and economically. Dr. Krambrich. “Both doctors as specialists have profile Hence the five founders decided to digitalise the based access to the relevant patient files from each analogous documentation of the patient file to simplify PC at all sites. -

Reactivating the Lake Junín Giant Frog Monitoring Program
December 2020 AMPHIBIAN SURVIVAL ALLIANCE NEWTSLETTER Got a story you want to share? Drop Candace an email today! [email protected] Stories from our partners around the world © Rogger Angel Moreno Lino Moreno Angel © Rogger Reactivating the Lake Junín Giant Frog monitoring program By Rogger Angel Moreno Lino, Luis and economy; businesses and NGOs In this way, ASA partner Grupo Castillo Roque and Roberto Elias have stopped their activities and RANA participated in a project for Piperis. Grupo RANA (Peru) and reduced their budgets among other the monitoring and surveillance of Denver Zoological Foundation (U.S.) things (Smith-Bingham & Harlharan, populations of the Lake Junín Giant [email protected] 2020; Crothers, 2020). However, Frog (Telmatobius macrostomus) solidarity among people and institu- and the Junín ‘Wanchas’ (Telma- The world is going through difficult tions has allowed activities to be tobius brachydactylus) in three times due to the COVID-19 pan- progressively reactivated, though protected natural areas (Junín Na- demic (WWF, 2020). Many people following rigorous biosecurity meas- tional Reserve, Historic Sanctuary of have been affected in their health ures. Chacamarca and Huayllay National Page 1 Sanctuary). These activities were by Pablo Miñano Lecaros. The park (CCPH), the presence of six adult led by the Denver Zoological Foun- rangers Winy Arias López, Eduardo frogs was recorded around 500 dation and funded by the National Ruiz and Duane Martínez supported meters from our monitoring point Geographic Society and followed the the activities. at the south of the Junín National biosecurity measures recommended As part of the preliminary results, Reserve. by the Ministry of Health of Peru we report three adults of the Junín It should be noted that the CCPH (D.S. -

Lauren A. O'connell
Lauren A. O’Connell Stanford University • 371 Serra Mall • Stanford, CA 94304 • phone 650-721-2768 [email protected] • oconnell.stanford.edu • ORCID: 0000-0002-2706-4077 __________________________________________________________________________________________ EDUCATION AND PROFESSIONAL EXPERIENCE Stanford University, Assistant Professor, DepartMent of Biology 2017 - present Harvard University, Bauer Fellow, FAS Center for SysteMs Biology 2012 - 2017 University of Texas at Austin, Ph.D. Cellular and Molecular Biology 2006 - 2011 Cornell University, B.S. Biological Sciences 2004 - 2006 Tarrant County College, A.A. 2002 - 2004 FUNDING National Science Foundation - Integrative Organismal SysteMs, $1,200,000 2019 - 2024 “CAREER: FroM ecology to neurobiology: spatial cognition in rainforest frogs” National Science Foundation - Integrative Organismal SysteMs, $1,600,000 2018 - 2021 “EDGE: Enabling functional genoMiCs tools in aMphibians” National Science Foundation - Integrative Organismal SysteMs, $800,000 2016 - 2020 “BioaccuMulation Mechanisms of defensive cheMicals in a poison frog” HellMan Faculty Scholar Award, $40,000 2018 - 2019 “Dietary tuning of infant social coMMunication” National Geographic Society CoMMittee for Research and Exploration, $18,600 2015 - 2016 “Convergent Evolution of Maternal Care in Poison Frogs” HONORS AND FELLOWSHIPS Kavli Fellow of the National AcadeMy of Sciences 2019 HellMan Faculty Fellow 2018 Frank A. Beach New Investigator Award 2018 L’Oreal USA Changing the Face of STEM Mentorship Grant 2016, 2018 L’Oreal USA For WoMen in Science Fellowship 2015 Adele Lewis Grant Fellowship froM Graduate WoMen in Science 2015 International Society for Neuroethology Konishi Neuroethology Research Award 2014 International Society for Neuroethology Capranica Prize 2013 International Society for Neuroethology Young Investigator Award 2012 Society for Social Neuroscience Early Career Award 2011 Society for Behavioral Neuroendocrinology Young Investigator Award 2011 UT-Austin WilliaM S. -

Rope Parasite” the Rope Parasite Parasites: Nearly Every Au�S�C Child I Ever Treated Proved to Carry a Significant Parasite Burden
Au#sm: 2015 Dietrich Klinghardt MD, PhD Infec4ons and Infestaons Chronic Infecons, Infesta#ons and ASD Infec4ons affect us in 3 ways: 1. Immune reac,on against the microbes or their metabolic products Treatment: low dose immunotherapy (LDI, LDA, EPD) 2. Effects of their secreted endo- and exotoxins and metabolic waste Treatment: colon hydrotherapy, sauna, intes4nal binders (Enterosgel, MicroSilica, chlorella, zeolite), detoxificaon with herbs and medical drugs, ac4vaon of detox pathways by solving underlying blocKages (methylaon, etc.) 3. Compe,,on for our micronutrients Treatment: decrease microbial load, consider vitamin/mineral protocol Lyme, Toxins and Epigene#cs • In 2000 I examined 10 au4s4c children with no Known history of Lyme disease (age 3-10), with the IgeneX Western Blot test – aer successful treatment. 5 children were IgM posi4ve, 3 children IgG, 2 children were negave. That is 80% of the children had clinical Lyme disease, none the history of a 4cK bite! • Why is it taking so long for au4sm-literate prac44oners to embrace the fact, that many au4s4c children have contracted Lyme or several co-infec4ons in the womb from an oVen asymptomac mother? Why not become Lyme literate also? • Infec4ons can be treated without the use of an4bio4cs, using liposomal ozonated essen4al oils, herbs, ozone, Rife devices, PEMF, colloidal silver, regular s.c injecons of artesunate, the Klinghardt co-infec4on cocKtail and more. • Symptomac infec4ons and infestaons are almost always the result of a high body burden of glyphosate, mercury and aluminum - against the bacKdrop of epigene4c injuries (epimutaons) suffered in the womb or from our ancestors( trauma, vaccine adjuvants, worK place related lead, aluminum, herbicides etc., electromagne4c radiaon exposures etc.) • Most symptoms are caused by a confused upregulated immune system (molecular mimicry) Toxins from a toxic environment enter our system through damaged boundaries and membranes (gut barrier, blood brain barrier, damaged endothelium, etc.). -

A Review of Chemical Defense in Poison Frogs (Dendrobatidae): Ecology, Pharmacokinetics, and Autoresistance
Chapter 21 A Review of Chemical Defense in Poison Frogs (Dendrobatidae): Ecology, Pharmacokinetics, and Autoresistance Juan C. Santos , Rebecca D. Tarvin , and Lauren A. O’Connell 21.1 Introduction Chemical defense has evolved multiple times in nearly every major group of life, from snakes and insects to bacteria and plants (Mebs 2002 ). However, among land vertebrates, chemical defenses are restricted to a few monophyletic groups (i.e., clades). Most of these are amphibians and snakes, but a few rare origins (e.g., Pitohui birds) have stimulated research on acquired chemical defenses (Dumbacher et al. 1992 ). Selective pressures that lead to defense are usually associated with an organ- ism’s limited ability to escape predation or conspicuous behaviors and phenotypes that increase detectability by predators (e.g., diurnality or mating calls) (Speed and Ruxton 2005 ). Defended organisms frequently evolve warning signals to advertise their defense, a phenomenon known as aposematism (Mappes et al. 2005 ). Warning signals such as conspicuous coloration unambiguously inform predators that there will be a substantial cost if they proceed with attack or consumption of the defended prey (Mappes et al. 2005 ). However, aposematism is likely more complex than the simple pairing of signal and defense, encompassing a series of traits (i.e., the apose- matic syndrome) that alter morphology, physiology, and behavior (Mappes and J. C. Santos (*) Department of Zoology, Biodiversity Research Centre , University of British Columbia , #4200-6270 University Blvd , Vancouver , BC , Canada , V6T 1Z4 e-mail: [email protected] R. D. Tarvin University of Texas at Austin , 2415 Speedway Stop C0990 , Austin , TX 78712 , USA e-mail: [email protected] L. -

Taxonomic Checklist of Amphibian Species Listed in the CITES
CoP17 Doc. 81.1 Annex 5 (English only / Únicamente en inglés / Seulement en anglais) Taxonomic Checklist of Amphibian Species listed in the CITES Appendices and the Annexes of EC Regulation 338/97 Species information extracted from FROST, D. R. (2015) "Amphibian Species of the World, an online Reference" V. 6.0 (as of May 2015) Copyright © 1998-2015, Darrel Frost and TheAmericanMuseum of Natural History. All Rights Reserved. Additional comments included by the Nomenclature Specialist of the CITES Animals Committee (indicated by "NC comment") Reproduction for commercial purposes prohibited. CoP17 Doc. 81.1 Annex 5 - p. 1 Amphibian Species covered by this Checklist listed by listed by CITES EC- as well as Family Species Regulation EC 338/97 Regulation only 338/97 ANURA Aromobatidae Allobates femoralis X Aromobatidae Allobates hodli X Aromobatidae Allobates myersi X Aromobatidae Allobates zaparo X Aromobatidae Anomaloglossus rufulus X Bufonidae Altiphrynoides malcolmi X Bufonidae Altiphrynoides osgoodi X Bufonidae Amietophrynus channingi X Bufonidae Amietophrynus superciliaris X Bufonidae Atelopus zeteki X Bufonidae Incilius periglenes X Bufonidae Nectophrynoides asperginis X Bufonidae Nectophrynoides cryptus X Bufonidae Nectophrynoides frontierei X Bufonidae Nectophrynoides laevis X Bufonidae Nectophrynoides laticeps X Bufonidae Nectophrynoides minutus X Bufonidae Nectophrynoides paulae X Bufonidae Nectophrynoides poyntoni X Bufonidae Nectophrynoides pseudotornieri X Bufonidae Nectophrynoides tornieri X Bufonidae Nectophrynoides vestergaardi -

The Path to the FAIR HANSA FAIR for More Than 600 Years, a Unique Network HANSA of Merchants Existed in Northern Europe
The path to the FAIR HANSA FAIR For more than 600 years, a unique network HANSA of merchants existed in Northern Europe. The cooperation of this consortium of merchants for the promotion of their foreign trade gave rise to an association of cities, to which around 200 coastal and inland cities belonged in the course of time. The Hanseatic League in the Middle Ages These cities were located in an area that today encom- passes seven European countries: from the Dutch Zui- derzee in the west to Baltic Estonia in the east, and from Sweden‘s Visby / Gotland in the north to the Cologne- Erfurt-Wroclaw-Krakow perimeter in the south. From this base, the Hanseatic traders developed a strong economic in uence, which during the 16th century extended from Portugal to Russia and from Scandinavia to Italy, an area that now includes 20 European states. Honest merchants – Fair Trade? Merchants, who often shared family ties to each other, were not always fair to producers and craftsmen. There is ample evidence of routine fraud and young traders in far- ung posts who led dissolute lives. It has also been proven that slave labor was used. ̇ ̆ Trading was conducted with goods that were typically regional, and sometimes with luxury goods: for example, wax and furs from Novgorod, cloth, silver, metal goods, salt, herrings and Chronology: grain from Hanseatic cities such as Lübeck, Münster or Dortmund 12th–14th Century - “Kaufmannshanse”. Establishment of Hanseatic trading posts (Hanseatic kontors) with common privi- leges for Low German merchants 14th–17th Century - “Städtehanse”. Cooperation between the Hanseatic cit- ies to defend their trade privileges and Merchants from di erent cities in di erent enforce common interests, especially at countries formed convoys and partnerships. -

RB 59 FAHRPLAN 2020 / 2021 Ankunft Und Anschlüsse
MONTAG - FREITAG Zug-Nummer 90317 90319 90365 90321 90367 90345 90391 90347 90349 90351 90353 90355 90357 90359 Aufgrund einer Baumaßnahme werden ab dem Dortmund Hbf ab 05:04 06:04 06:34 07:04 07:34 19:04 19:34 20:04 21:04 22:04 23:04 00:04 01:04 02:04 02. Juli 2021 von Montag bis Samstag die Züge Dortmund Signal-Iduna-Park 05:12 06:12 06:42 07:12 07:42 19:12 19:42 20:12 21:12 22:12 23:12 00:12 01:12 02:12 Fahrplanauskünfte Dortmund-Hörde 05:15 06:15 06:46 07:15 07:46 19:15 19:46 20:15 21:15 22:15 23:15 00:15 01:15 02:15 des Zwischentakts (Abfahrt Dortmund Hbf zur Dortmund-Aplerbeck 05:19 06:19 06:50 07:19 07:50 19:19 19:50 20:19 21:19 22:19 23:19 00:19 01:19 02:19 Minute :34) mit abweichenden Fahrtzeiten zwi- Dortmund-Sölde 05:23 06:23 06:53 07:23 07:53 19:23 19:53 20:23 21:23 22:23 23:23 00:23 01:23 02:23 schen Dortmund Hbf - Soest und ohne Halt in Aktuelle Fahrzeiten und Informationen Holzwickede / DO Flughafen 05:27 06:27 06:57 07:27 07:57 19:27 19:57 20:27 21:27 22:27 23:27 00:27 01:27 02:27 Dortmund-Aplerbeck verkehren. stehen Ihnen auf unserer Webseite unter Unna an 05:31 06:31 07:01 07:31 08:01 19:31 20:01 20:31 21:31 22:31 23:31 00:31 01:31 02:32 eurobahn.de/abfahrtsinfos zur Verfügung. -

Dortmund – Unna
Unna – Unna-Königsborn – Dortmund-Wickede – Dortmund Stadthaus – Dortmund-Dorstfeld – Dortmund-Lütgendortmund Unna 4 - Unna-Königsborn - Dortmund-Wickede - Dortmund Stadthaus - Dortmund-Dorstfeld - Dortmund-Lütgendortmund S 4 Unna - Unna-Königsborn - Dortmund-Wickede - Dortmund Stadthaus - Dortmund-Dorstfeld - Dortmund-Lütgendortmund Montag bis Freitag S 4 Linie S 4 S 4 S 4 S 4 S 4 S 4 S 4 S 4 S 4 S 4 S 4 S 4 S 4 S 4 S 4 S 4 S 4 S 4 S 4 S 4 S 4 S 4 S 4 S 4 S 4 S 4 S 4 S 4 S 4 S 4 S 4 S 4 S 4 S 4 S 4 S 4 S 4 S 4 S 4 S 4 S 4 S 4 S 4 S 4 S 4 S 4 S 4 S 4 S 4 S 4 S 4 S 4 S 4 S 4 S 4 S 4 Gültig ab 13. -
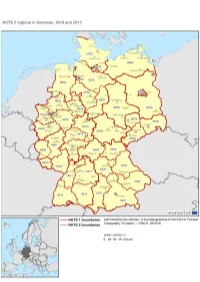
Nuts-Map-DE.Pdf
GERMANY NUTS 2013 Code NUTS 1 NUTS 2 NUTS 3 DE1 BADEN-WÜRTTEMBERG DE11 Stuttgart DE111 Stuttgart, Stadtkreis DE112 Böblingen DE113 Esslingen DE114 Göppingen DE115 Ludwigsburg DE116 Rems-Murr-Kreis DE117 Heilbronn, Stadtkreis DE118 Heilbronn, Landkreis DE119 Hohenlohekreis DE11A Schwäbisch Hall DE11B Main-Tauber-Kreis DE11C Heidenheim DE11D Ostalbkreis DE12 Karlsruhe DE121 Baden-Baden, Stadtkreis DE122 Karlsruhe, Stadtkreis DE123 Karlsruhe, Landkreis DE124 Rastatt DE125 Heidelberg, Stadtkreis DE126 Mannheim, Stadtkreis DE127 Neckar-Odenwald-Kreis DE128 Rhein-Neckar-Kreis DE129 Pforzheim, Stadtkreis DE12A Calw DE12B Enzkreis DE12C Freudenstadt DE13 Freiburg DE131 Freiburg im Breisgau, Stadtkreis DE132 Breisgau-Hochschwarzwald DE133 Emmendingen DE134 Ortenaukreis DE135 Rottweil DE136 Schwarzwald-Baar-Kreis DE137 Tuttlingen DE138 Konstanz DE139 Lörrach DE13A Waldshut DE14 Tübingen DE141 Reutlingen DE142 Tübingen, Landkreis DE143 Zollernalbkreis DE144 Ulm, Stadtkreis DE145 Alb-Donau-Kreis DE146 Biberach DE147 Bodenseekreis DE148 Ravensburg DE149 Sigmaringen DE2 BAYERN DE21 Oberbayern DE211 Ingolstadt, Kreisfreie Stadt DE212 München, Kreisfreie Stadt DE213 Rosenheim, Kreisfreie Stadt DE214 Altötting DE215 Berchtesgadener Land DE216 Bad Tölz-Wolfratshausen DE217 Dachau DE218 Ebersberg DE219 Eichstätt DE21A Erding DE21B Freising DE21C Fürstenfeldbruck DE21D Garmisch-Partenkirchen DE21E Landsberg am Lech DE21F Miesbach DE21G Mühldorf a. Inn DE21H München, Landkreis DE21I Neuburg-Schrobenhausen DE21J Pfaffenhofen a. d. Ilm DE21K Rosenheim, Landkreis DE21L Starnberg DE21M Traunstein DE21N Weilheim-Schongau DE22 Niederbayern DE221 Landshut, Kreisfreie Stadt DE222 Passau, Kreisfreie Stadt DE223 Straubing, Kreisfreie Stadt DE224 Deggendorf DE225 Freyung-Grafenau DE226 Kelheim DE227 Landshut, Landkreis DE228 Passau, Landkreis DE229 Regen DE22A Rottal-Inn DE22B Straubing-Bogen DE22C Dingolfing-Landau DE23 Oberpfalz DE231 Amberg, Kreisfreie Stadt DE232 Regensburg, Kreisfreie Stadt DE233 Weiden i. -

Summary Record of the 26Th Meeting of the Animals Committee
Original language: English AC26 summary record CONVENTION ON INTERNATIONAL TRADE IN ENDANGERED SPECIES OF WILD FAUNA AND FLORA ____________ Twenty-sixth meeting of the Animals Committee Geneva (Switzerland), 15-20 March 2012 and Dublin (Ireland), 22-24 March 2012 SUMMARY RECORD Animals Committee matters 1. Opening of the meeting The Chair opened the meeting and welcomed all participants, before giving the floor to the Secretary- General, who also welcomed everyone and introduced new members of the Secretariat's scientific team (Mr De Meulenaer and Ms Kwitsinskaia) and enforcement team (Ms Garcia Ferreira, Ms Jonsson and Mr van Rensburg). He wished the Committee well in its deliberations. The Chair thanked the Secretary-General and invited suggestions as to how the Conference of the Parties could establish stronger measures to support the Committee as well as export countries, which deserved particular assistance. No other intervention was made during discussion of this item.1 2. Rules of Procedure The Secretariat introduced document AC26 Doc. 2 and proposed amending Rule 22 as follows: “On request, the Secretariat shall distribute printed and translated documents...”. The Secretariat explained that most members regularly indicated that they did not need printed copies and that this proposal was made to reduce costs. Although not opposed to the change in principle, a Party regretted that the suggestion had not been presented in the document, which would have given Parties time to consider it, and was concerned that this unannounced proposal might create a precedent. Another Party asked a question on the procedure to accept observers, but the Chair invited it to raise this topic under agenda item 4 on Admission of observers.