Spatial and Temporal Dynamics in the Development of Invading Cynipid Communities in Britain
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Checklist of British and Irish Hymenoptera - Cynipoidea
Biodiversity Data Journal 5: e8049 doi: 10.3897/BDJ.5.e8049 Taxonomic Paper Checklist of British and Irish Hymenoptera - Cynipoidea Mattias Forshage‡, Jeremy Bowdrey§, Gavin R. Broad |, Brian M. Spooner¶, Frank van Veen# ‡ Swedish Museum of Natural History, Stockholm, Sweden § Colchester and Ipswich Museums, Colchester, United Kingdom | The Natural History Museum, London, United Kingdom ¶ Royal Botanic Gardens, Kew, Richmond, United Kingdom # University of Exeter, Penryn, United Kingdom Corresponding author: Gavin R. Broad ([email protected]) Academic editor: Pavel Stoev Received: 05 Feb 2016 | Accepted: 06 Mar 2017 | Published: 09 Mar 2017 Citation: Forshage M, Bowdrey J, Broad G, Spooner B, van Veen F (2017) Checklist of British and Irish Hymenoptera - Cynipoidea. Biodiversity Data Journal 5: e8049. https://doi.org/10.3897/BDJ.5.e8049 Abstract Background The British and Irish checklist of Cynipoidea is revised, considerably updating the last complete checklist published in 1978. Disregarding uncertain identifications, 220 species are now known from Britain and Ireland, comprising 91 Cynipidae (including two established non-natives), 127 Figitidae and two Ibaliidae. New information One replacement name is proposed, Kleidotoma thomsoni Forshage, for the secondary homonym Kleidotoma tetratoma Thomson, 1861 (nec K. tetratoma (Hartig, 1841)). © Forshage M et al. This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. 2 Forshage M et al Introduction This paper continues the series of updated British and Irish Hymenoptera checklists that started with Broad and Livermore (2014a), Broad and Livermore (2014b), Liston et al. -

Quercus Cerris
Quercus cerris Quercus cerris in Europe: distribution, habitat, usage and threats D. de Rigo, C. M. Enescu, T. Houston Durrant, G. Caudullo Turkey oak (Quercus cerris L.) is a deciduous tree native to southern Europe and Asia Minor, and a dominant species in the mixed forests of the Mediterranean basin. Turkey oak is a representative of section Cerris, a particular section within the genus Quercus which includes species for which the maturation of acorns occurs in the second year. Quercus cerris L., commonly known as Turkey oak, is a large fast-growing deciduous tree species growing to 40 m tall with 1 Frequency a trunk up to 1.5-2 m diameter , with a well-developed root < 25% system2. It can live for around 120-150 years3. The bark is 25% - 50% 50% - 75% mauve-grey and deeply furrowed with reddish-brown or orange > 75% bark fissures4, 5. Compared with other common oak species, e.g. Chorology Native sessile oak (Quercus petraea) and pedunculate oak (Quercus Introduced robur), the wood is inferior, and only useful for rough work such as shuttering or fuelwood1. The leaves are dark green above and grey-felted underneath6; they are variable in size and shape but are normally 9-12 cm long and 3-5 cm wide, with 7-9 pairs of triangular lobes6. The leaves turn yellow to gold in late autumn and drop off or persist in the crown until the next spring, especially on young trees3. The twigs are long and pubescent, grey or olive-green, with lenticels. The buds, which are concentrated Large shade tree in agricultural area near Altamura (Bari, South Italy). -

The Dorset Heath 2013 So Once Again You Have Me As Editor
NewsletterThe ofD theo Dorsetrset Flora H eGroupath 201 4 Chairman and VC9 Recorder Robin Walls; Secretary Laurence Taylor Editorial: John Newbould It would appear that the group had no complaints about the layout and content of the Dorset Heath 2013 so once again you have me as editor. The year was somewhat difficult for me as somehow, whenever I had to leave the room in Yorkshire Naturalists’ Union committee meetings in 2011, they managed to appoint me President for 2013 resulting in extra commitments in that county. During April 2013, Dorset hosted the National Forum for Biological Recording’s annual conference at the R.N.L.I. College at Poole. What a fabulous conference venue and the overnight accommodation was excellent. NFBR then joined Dorset naturalists with a joint meeting based at Studland helping to survey for the Cyril Diver project. Once again, duties took me away as I seem to be the conference administrator. The Flora Group had an interesting year, with variable numbers at field meetings. Never-the-less some important recording has been achieved including members engaging with recording bryophytes for the first time, one meeting to record fungi near Hardy’s Cottage, which thanks to the expertise of Bryan Edwards was very successful. We also had a few members try their hand at lichen recording In June 2014, I have been tasked by the Linnean Society to organise their annual field trip, which will be in June starting with a day on Portland and Chesil on the Saturday with Ballard Down and Studland on the Sunday. -
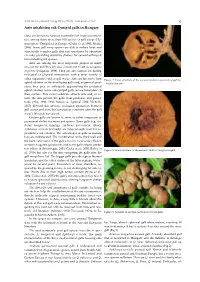
Ants Inhabiting Oak Cynipid Galls in Hungary
North-Western Journal of Zoology 2020, vol.16 (1) - Correspondence: Notes 95 Ants inhabiting oak Cynipid galls in Hungary Oaks are known to harbour extremely rich insect communi- ties, among them more than 100 species of gall wasps (Hy- menoptera: Cynipidae) in Europe (Csóka et al. 2005, Melika 2006). Some gall wasp species are able to induce large and structurally complex galls that can sometimes be abundant on oaks, providing attractive shelters for several arthropod taxa including ant species. Ants are among the most important players in many ecosystems and they are also considered to act as ecosystem engineers (Folgarait, 1998). They are also famous for having ecological or physical interactions with a great variety of other organisms, such as gall wasps. Ants are known to tend Figure 1. Inner structure of the asexual Andricus quercustozae gall in- aphid colonies on the developing galls and, as general pred- habited by ants. ators, they prey on arthropods approaching the protected aphid colonies. Some oak cynipid galls secrete honeydew on their surface. This sweet substrate attracts ants and, in re- turn, the ants protect the galls from predators and parasi- toids (Abe, 1988, 1992; Inouye & Agrawal 2004; Nicholls, 2017). Beyond this obvious ecological interaction between gall wasps and ants, this association continues after the gall wasp’s life cycle has ceased. Certain galls are known to serve as either temporary or permanent shelter for many ant species. Some galls (e.g. An- dricus hungaricus (Hartig), Andricus quercustozae (Bosc), Aphelonyx cerricola (Giraud)) are large enough even for re- productive ant colonies. The advantages of galls as nesting logs are multifaceted. -

The Structure of Cynipid Oak Galls: Patterns in the Evolution of an Extended Phenotype
The structure of cynipid oak galls: patterns in the evolution of an extended phenotype Graham N. Stone1* and James M. Cook2 1Department of Zoology, University of Oxford, South Parks Road, Oxford OX1 3PS, UK ([email protected]) 2Department of Biology, Imperial College, Silwood Park, Ascot, Berkshire SL5 7PY, UK Galls are highly specialized plant tissues whose development is induced by another organism. The most complex and diverse galls are those induced on oak trees by gallwasps (Hymenoptera: Cynipidae: Cyni- pini), each species inducing a characteristic gall structure. Debate continues over the possible adaptive signi¢cance of gall structural traits; some protect the gall inducer from attack by natural enemies, although the adaptive signi¢cance of others remains undemonstrated. Several gall traits are shared by groups of oak gallwasp species. It remains unknown whether shared traits represent (i) limited divergence from a shared ancestral gall form, or (ii) multiple cases of independent evolution. Here we map gall character states onto a molecular phylogeny of the oak cynipid genus Andricus, and demonstrate three features of the evolution of gall structure: (i) closely related species generally induce galls of similar structure; (ii) despite this general pattern, closely related species can induce markedly di¡erent galls; and (iii) several gall traits (the presence of many larval chambers in a single gall structure, surface resins, surface spines and internal air spaces) of demonstrated or suggested adaptive value to the gallwasp have evolved repeatedly. We discuss these results in the light of existing hypotheses on the adaptive signi¢cance of gall structure. Keywords: galls; Cynipidae; enemy-free space; extended phenotype; Andricus layers of woody or spongy tissue, complex air spaces within 1. -

Hymenoptera: Eulophidae) 321-356 ©Entomofauna Ansfelden/Austria; Download Unter
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Entomofauna Jahr/Year: 2007 Band/Volume: 0028 Autor(en)/Author(s): Yefremova Zoya A., Ebrahimi Ebrahim, Yegorenkova Ekaterina Artikel/Article: The Subfamilies Eulophinae, Entedoninae and Tetrastichinae in Iran, with description of new species (Hymenoptera: Eulophidae) 321-356 ©Entomofauna Ansfelden/Austria; download unter www.biologiezentrum.at Entomofauna ZEITSCHRIFT FÜR ENTOMOLOGIE Band 28, Heft 25: 321-356 ISSN 0250-4413 Ansfelden, 30. November 2007 The Subfamilies Eulophinae, Entedoninae and Tetrastichinae in Iran, with description of new species (Hymenoptera: Eulophidae) Zoya YEFREMOVA, Ebrahim EBRAHIMI & Ekaterina YEGORENKOVA Abstract This paper reflects the current degree of research of Eulophidae and their hosts in Iran. A list of the species from Iran belonging to the subfamilies Eulophinae, Entedoninae and Tetrastichinae is presented. In the present work 47 species from 22 genera are recorded from Iran. Two species (Cirrospilus scapus sp. nov. and Aprostocetus persicus sp. nov.) are described as new. A list of 45 host-parasitoid associations in Iran and keys to Iranian species of three genera (Cirrospilus, Diglyphus and Aprostocetus) are included. Zusammenfassung Dieser Artikel zeigt den derzeitigen Untersuchungsstand an eulophiden Wespen und ihrer Wirte im Iran. Eine Liste der für den Iran festgestellten Arten der Unterfamilien Eu- lophinae, Entedoninae und Tetrastichinae wird präsentiert. Mit vorliegender Arbeit werden 47 Arten in 22 Gattungen aus dem Iran nachgewiesen. Zwei neue Arten (Cirrospilus sca- pus sp. nov. und Aprostocetus persicus sp. nov.) werden beschrieben. Eine Liste von 45 Wirts- und Parasitoid-Beziehungen im Iran und ein Schlüssel für 3 Gattungen (Cirro- spilus, Diglyphus und Aprostocetus) sind in der Arbeit enthalten. -

National Oak Gall Wasp Survey
ational Oak Gall Wasp Survey – mapping with parabiologists in Finland Bess Hardwick Table of Contents 1. Introduction ................................................................................................................. 2 1.1. Parabiologists in data collecting ............................................................................. 2 1.2. Oak cynipid gall wasps .......................................................................................... 3 1.3. Motivations and objectives .................................................................................... 4 2. Material and methods ................................................................................................ 5 2.1. The volunteers ........................................................................................................ 5 2.2. Sampling ................................................................................................................. 6 2.3. Processing of samples ............................................................................................ 7 2.4. Data selection ........................................................................................................ 7 2.5. Statistical analyses ................................................................................................. 9 3. Results ....................................................................................................................... 10 3.1. Sampling success ................................................................................................. -

The Population Biology of Oak Gall Wasps (Hymenoptera:Cynipidae)
5 Nov 2001 10:11 AR AR147-21.tex AR147-21.SGM ARv2(2001/05/10) P1: GSR Annu. Rev. Entomol. 2002. 47:633–68 Copyright c 2002 by Annual Reviews. All rights reserved THE POPULATION BIOLOGY OF OAK GALL WASPS (HYMENOPTERA:CYNIPIDAE) Graham N. Stone,1 Karsten Schonrogge,¨ 2 Rachel J. Atkinson,3 David Bellido,4 and Juli Pujade-Villar4 1Institute of Cell, Animal, and Population Biology, University of Edinburgh, The King’s Buildings, West Mains Road, Edinburgh EH9 3JT, United Kingdom; e-mail: [email protected] 2Center of Ecology and Hydrology, CEH Dorset, Winfrith Technology Center, Winfrith Newburgh, Dorchester, Dorset DT2 8ZD, United Kingdom; e-mail: [email protected] 3Center for Conservation Science, Department of Biology, University of Stirling, Stirling FK9 4LA, United Kingdom; e-mail: [email protected] 4Departamento de Biologia Animal, Facultat de Biologia, Universitat de Barcelona, Avenida Diagonal 645, 08028 Barcelona, Spain; e-mail: [email protected] Key Words cyclical parthenogenesis, host alternation, food web, parasitoid, population dynamics ■ Abstract Oak gall wasps (Hymenoptera: Cynipidae, Cynipini) are characterized by possession of complex cyclically parthenogenetic life cycles and the ability to induce a wide diversity of highly complex species- and generation-specific galls on oaks and other Fagaceae. The galls support species-rich, closed communities of inquilines and parasitoids that have become a model system in community ecology. We review recent advances in the ecology of oak cynipids, with particular emphasis on life cycle characteristics and the dynamics of the interactions between host plants, gall wasps, and natural enemies. We assess the importance of gall traits in structuring oak cynipid communities and summarize the evidence for bottom-up and top-down effects across trophic levels. -

Butlleti 71.P65
Butll. Inst. Cat. Hist. Nat., 71: 83-95. 2003 ISSN: 1133-6889 GEA, FLORA ET FAUNA The life cycle of Andricus hispanicus (Hartig, 1856) n. stat., a sibling species of A. kollari (Hartig, 1843) (Hymenoptera: Cynipidae) Juli Pujade-Villar*, Roger Folliot** & David Bellido* Rebut: 28.07.03 Acceptat: 01.12.03 Abstract and so we consider A. mayeti and A. niger to be junior synonyms of A. hispanicus. Finally, possible causes of the speciation of A. kollari and The marble gallwasp, Andricus kollari, common A. hispanicus are discussed. and widespread in the Western Palaeartic, is known for the conspicuous globular galls caused by the asexual generations on the buds of several KEY WORDS: Cynipidae, Andricus, A. kollari, A. oak species. The sexual form known hitherto, hispanicus, biological cycle, sibling species, formerly named Andricus circulans, makes small sexual form, speciation, distribution, morphology, gregarious galls on the buds of Turkey oak, A. mayeti, A. burgundus. Quercus cerris; this oak, however, is absent from the Iberian Peninsula, where on the other hand the cork oak, Q. suber, is present. Recent genetic studies show the presence of two different Resum populations or races with distribution patterns si- milar to those of Q. cerris and Q. suber. We present new biological and morphological Cicle biològic d’Andricus hispanicus (Hartig, evidence supporting the presence of a sibling 1856) una espècie bessona d’A. kollari (Hartig, species of A. kollari in the western part of its 1843) (Hymenoptera: Cynipidae) range (the Iberian Peninsula, southern France and North Africa), Andricus hispanicus n. stat.. Biological and morphological differences separating these Andricus kollari és una espècie molt comuna dis- two species from other closely related ones are tribuida a l’oest del paleartic coneguda per la given and the new sexual form is described for the gal·la globular i relativament gran de la generació first time. -

Preliminary Cladistics and Review of Hemiptarsenus Westwood and Sympiesis Förster (Hymenoptera, Eulophidae) in Hungary
Zoological Studies 42(2): 307-335 (2003) Preliminary Cladistics and Review of Hemiptarsenus Westwood and Sympiesis Förster (Hymenoptera, Eulophidae) in Hungary Chao-Dong Zhu and Da-Wei Huang* Parasitoid Group, Institute of Zoology, Chinese Academy of Sciences, Beijing 100080, China (Accepted January 7, 2003) Chao-Dong Zhu and Da-Wei Huang (2003) Preliminary cladistics and review of Hemiptarsenus Westwood and Sympiesis Förster (Hymenoptera, Eulophidae) in Hungary. Zoological Studies 42(2): 307-335. A cladistic analysis of known species of both Hemiptarsenus Westwood and Sympiesis Förster (Hymenoptera: Eulophidae) in Hungary was carried out based on 176 morphological characters from adults. Three most-parsi- monious trees (MPTs) were produced, strictly consensused, and rerooted. Monophyly of Sympiesis was sup- ported by all 3 MPTs. A review of the genera Hemiptarsenus Westwood and Sympiesis Förster was made based on the results of the cladistic analysis. Sympiesis petiolata was transferred into Hemiptarsenus. Several other species in both Hemiptarsenus and Sympiesis were removed from the synonymy lists of different species and reinstated. http://www.sinica.edu.tw/zool/zoolstud/42.2/307.pdf Key words: Taxonomy, Cladistics, Hemiptarsenus, Sympiesis, Hungary. W orking on Chinese fauna of the deposited at the Hungarian Natural History Chalcidoidea (Zhu et al. 1999 2000a, Zhu and Museum (HNHM); careful re-examination of their Huang 2000a b c 2001a b 2002a b c, Xiao and materials is needed to update knowledge of this Huang 2001a b c d e), we have found many taxa group. In May 2001, the senior author was sup- which occur in North China that have been also ported by the National Scientific Fund of China reported from Europe. -

Fossil Oak Galls Preserve Ancient Multitrophic Interactions
Edinburgh Research Explorer Fossil oak galls preserve ancient multitrophic interactions Citation for published version: Stone, GN, van der Ham, RWJM & Brewer, JG 2008, 'Fossil oak galls preserve ancient multitrophic interactions', Proceedings of the Royal Society B-Biological Sciences, vol. 275, no. 1648, pp. 2213-2219. https://doi.org/10.1098/rspb.2008.0494 Digital Object Identifier (DOI): 10.1098/rspb.2008.0494 Link: Link to publication record in Edinburgh Research Explorer Document Version: Publisher's PDF, also known as Version of record Published In: Proceedings of the Royal Society B-Biological Sciences Publisher Rights Statement: Free in PMC. General rights Copyright for the publications made accessible via the Edinburgh Research Explorer is retained by the author(s) and / or other copyright owners and it is a condition of accessing these publications that users recognise and abide by the legal requirements associated with these rights. Take down policy The University of Edinburgh has made every reasonable effort to ensure that Edinburgh Research Explorer content complies with UK legislation. If you believe that the public display of this file breaches copyright please contact [email protected] providing details, and we will remove access to the work immediately and investigate your claim. Download date: 01. Oct. 2021 Proc. R. Soc. B (2008) 275, 2213–2219 doi:10.1098/rspb.2008.0494 Published online 17 June 2008 Fossil oak galls preserve ancient multitrophic interactions Graham N. Stone1,*, Raymond W. J. M. van der Ham2 and Jan G. Brewer3 1Institute of Evolutionary Biology, University of Edinburgh, West Mains Road, Edinburgh EH9 3JT, UK 2Nationaal Herbarium Nederland, Universiteit Leiden, PO Box 9514, 2300 RA Leiden, The Netherlands 3Hogebroeksweg 32, 8102 RK Raalte, The Netherlands Trace fossils of insect feeding have contributed substantially to our understanding of the evolution of insect–plant interactions. -

Naturalist April 2013 1082
April 2013 Volume 138 Number 1082 Yorkshire Union The Naturalist Vol. 138 No. 1082 April 2013 Contents Page Editorial 1 John Newbould: President of the YNU 2012-2013 2 Aqua�c plants in Yorkshire canals R. Goulder 4 An interes�ng plant gall on Gorse Derek Parkinson 16 Andricus gemmeus – a new gall for Yorkshire Tom Higginbo�om 17 A provisional Vascular Plant Red Data List for VC63 ‐ an evalua�on of current status 18 G.T.D. Wilmore The Gledhow Valley Woods Nest Box Scheme Mar�n Calvert 31 Onset of Summer Plumage in Black‐headed Gulls at Doncaster Lakeside, based on 35 field observa�ons January to March 2012* Colin A. Howes and John A. Porter Notes on Sowerby’s Beaked Whale strandings on the Yorkshire coast* 38 D.E. Whi�aker Seals at Teesmouth: a historical review Colin A. Howes and Robert Woods 42 Rosemary Beetle Chrysolina americana ‐ a new beetle record for Mid‐west Yorkshire 49 G. Boyd Field Note ‐ Rhododendron lea�opper in VC64 Mark Darwell and John Bowers 50 Recording in VC65 July 2012 John Newbould, Adrian Norris and Bill Ely 52 Botanical Report for 2012 Phyl Abbo� 62 YNU Excursions 2013 70 Project: The Yorkshire Flat Hedgehog Survey Colin A. Howes 78 Project: Parasi�sm of Coleophora serratella Derek Parkinson 79 YNU Calendar April ‐ August 2013 80 Book review: p77 YNU No�ce: p79 An asterix* indicates a peer‐reviewed paper Front cover: Hound’s‐tongue Cynoglossum officinale, one of the rare na�ve plants proposed for VC63’s Red Data List of plants (see p21).