Histopathology and Host Range Studies of the Redwood Nematode Rhizonema Sequoiae 1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Scientific Name Species Common Name Abies Lasiocarpa FIR Subalpine Acacia Macracantha ACACIA Long-Spine
Scientific Name Species Common Name Abies lasiocarpa FIR Subalpine Acacia macracantha ACACIA Long-spine Acacia roemeriana CATCLAW Roemer Acer grandidentatum MAPLE Canyon Acer nigrum MAPLE Black Acer platanoides MAPLE Norway Acer saccharinum MAPLE Silver Aesculus pavia BUCKEYE Red Aesculus sylvatica BUCKEYE Painted Ailanthus altissima AILANTHUS Tree-of-heaven Albizia julibrissin SILKTREE Mimosa Albizia lebbek LEBBEK Lebbek Alnus iridis ssp. sinuata ALDER Sitka Alnus maritima ALDER Seaside Alvaradoa amorphoides ALVARADOA Mexican Amelanchier laevis SERVICEBERRY Allegheny Amyris balsamifera TORCHWOOD Balsam Annona squamosa SUGAR-APPLE NA Araucaria cunninghamii ARAUCARIA Cunningham Arctostaphylos glauca MANZANITA Bigberry Asimina obovata PAWPAW Bigflower Bourreria radula STRONGBACK Rough Brasiliopuntia brasiliensis PRICKLY-PEAR Brazilian Bursera simaruba GUMBO-LIMBO NA Caesalpinia pulcherrima FLOWERFENCE NA Capparis flexuosa CAPERTREE Limber CRUCIFIXION- Castela emoryi THORN NA Casuarina equisetifolia CASUARINA Horsetail Ceanothus arboreus CEANOTHUS Feltleaf Ceanothus spinosus CEANOTHUS Greenbark Celtis lindheimeri HACKBERRY Lindheimer Celtis occidentalis HACKBERRY Common Cephalanthus occidentalis BUTTONBUSH Common Cercis canadensis REDBUD Eastern Cercocarpus traskiae CERCOCARPUS Catalina Chrysophyllum oliviforme SATINLEAF NA Citharexylum berlandieri FIDDLEWOOD Berlandier Citrus aurantifolia LIME NA Citrus sinensis ORANGE Orange Coccoloba uvifera SEAGRAPE NA Colubrina arborescens COLUBRINA Coffee Colubrina cubensis COLUBRINA Cuba Condalia globosa -

CDFG Natural Communities List
Department of Fish and Game Biogeographic Data Branch The Vegetation Classification and Mapping Program List of California Terrestrial Natural Communities Recognized by The California Natural Diversity Database September 2003 Edition Introduction: This document supersedes all other lists of terrestrial natural communities developed by the Natural Diversity Database (CNDDB). It is based on the classification put forth in “A Manual of California Vegetation” (Sawyer and Keeler-Wolf 1995 and upcoming new edition). However, it is structured to be compatible with previous CNDDB lists (e.g., Holland 1986). For those familiar with the Holland numerical coding system you will see a general similarity in the upper levels of the hierarchy. You will also see a greater detail at the lower levels of the hierarchy. The numbering system has been modified to incorporate this richer detail. Decimal points have been added to separate major groupings and two additional digits have been added to encompass the finest hierarchal detail. One of the objectives of the Manual of California Vegetation (MCV) was to apply a uniform hierarchical structure to the State’s vegetation types. Quantifiable classification rules were established to define the major floristic groups, called alliances and associations in the National Vegetation Classification (Grossman et al. 1998). In this document, the alliance level is denoted in the center triplet of the coding system and the associations in the right hand pair of numbers to the left of the final decimal. The numbers of the alliance in the center triplet attempt to denote relationships in floristic similarity. For example, the Chamise-Eastwood Manzanita alliance (37.106.00) is more closely related to the Chamise- Cupleaf Ceanothus alliance (37.105.00) than it is to the Chaparral Whitethorn alliance (37.205.00). -

CHARACTERISTICS of CALIFORNIA ANNUAL GRASSLANDS VEGETATION SERIES on TSR
CHARACTERISTICS OF CALIFORNIA ANNUAL GRASSLANDS VEGETATION SERIES on TSR DOMINANT SPECIES IN CALIFORNIA GRASSLAND SERIES Holcus Lanatus Velvet Grass Introduced Anthoxanthum odoratum Sweet Vernal Grass Introduced Briza Maxima Rattlesnake Grass Introduced Danthonia sp. Oat Grasses Native Deschampsia sp. Hair Grasses Native COMMONLY ASSOCIATED SPECIES by vegetation types TREES Rare, usually as isolated seedlings SHRUBS Isolated, transitioning Baccharis pilularis Coyote brush Rhamnus californica Coffee berry Lupinus arborescens Yellow or blue lupine Yellow Introduced HERBS AND GRASSES Eschscholzia californica California poppy, coastal (yellow petals) Iris douglasiana Douglas iris Sisyrinchium bellum Yellow-eyed grass Various genera Thistles Introduced CHARACTERISTICS OF PACIFIC REEDGRASS VEGETATION SERIES DOMINANT SPECIES IN PACIFIC REED GRASS SERIES Calimagrostis nutkaensis Pacific Reed Grass COMMONLY ASSOCIATED SPECIES listed by vegetation types TREES None SHRUBS Myrica californica Wax Myrtle Rosa sp Wild Rose HERBS AND GRASSES Rubus ursinus California blackberry Holcus Lanatus Velvet Grass Pteridium aquilinum Bracken Juncus and Carex sp Rushes and Sedgees Veratrum fimbriatum Corn Lily CHARACTERISTICS OF COYOTE BRUSH VEGETATION SERIES DOMINANT SPECIES Baccharis pilularis Coyote brush COMMONLY ASSOCIATED SPECIES listed by vegetation types TREES Occasional as emerging forest Pseutotsuga menziesii Douglas fir Pinus muricata Bishop Pine SHRUBS Rubus ursinus California blackberry Myrica californica Wax myrtle Toxicodendron diversiloba Poison -

Conifer Communities of the Santa Cruz Mountains and Interpretive
UNIVERSITY OF CALIFORNIA, SANTA CRUZ CALIFORNIA CONIFERS: CONIFER COMMUNITIES OF THE SANTA CRUZ MOUNTAINS AND INTERPRETIVE SIGNAGE FOR THE UCSC ARBORETUM AND BOTANIC GARDEN A senior internship project in partial satisfaction of the requirements for the degree of BACHELOR OF ARTS in ENVIRONMENTAL STUDIES by Erika Lougee December 2019 ADVISOR(S): Karen Holl, Environmental Studies; Brett Hall, UCSC Arboretum ABSTRACT: There are 52 species of conifers native to the state of California, 14 of which are endemic to the state, far more than any other state or region of its size. There are eight species of coniferous trees native to the Santa Cruz Mountains, but most people can only name a few. For my senior internship I made a set of ten interpretive signs to be installed in front of California native conifers at the UCSC Arboretum and wrote an associated paper describing the coniferous forests of the Santa Cruz Mountains. Signs were made using the Arboretum’s laser engraver and contain identification and collection information, habitat, associated species, where to see local stands, and a fun fact or two. While the physical signs remain a more accessible, kid-friendly format, the paper, which will be available on the Arboretum website, will be more scientific with more detailed information. The paper will summarize information on each of the eight conifers native to the Santa Cruz Mountains including localized range, ecology, associated species, and topics pertaining to the species in current literature. KEYWORDS: Santa Cruz, California native plants, plant communities, vegetation types, conifers, gymnosperms, environmental interpretation, UCSC Arboretum and Botanic Garden I claim the copyright to this document but give permission for the Environmental Studies department at UCSC to share it with the UCSC community. -

Redwood Guidelines
Redwood Guidelines Introduction and Purpose The Conservation Committee strives to protect heritage and significant sized trees that are growing in appropriate natural habitats where they thrive without human intervention. Sequoia sempervirens, or Coast Redwoods, are iconic California native plants that are among the tallest and longest living of all trees. These trees once covered 1.6 million acres of California in 1850, but now more than 95% of the old growth forest is gone, lost to indiscriminate logging, especially during the gold rush. Redwoods are admirable trees that are familiar in the Portola Valley landscape and we are fortunate that this unique tree can thrive in our community. Like most native plants, redwoods thrive naturally in habitats that are appropriate to their needs. Specifically, they need both summer and winter fog and adequate rainfall, which occurs in a narrow coastal belt between the 42nd and 36th degree North latitudes. Portola Valley is at 37.3 degrees North. Humans can alter habitats in such ways as to allow almost any plant to grow, even if that species would not normally be found in that location. Since redwoods require a constant supply of water in the summer, they do not grow naturally in the oak woodlands and other dry land communities in the hills on the bay side of our valley where fog drip is not as common. Redwoods can only stay healthy and alive in those habitats with the human intervention of summer watering. The purpose of these guidelines is to provide current and future homeowners with information on where it is appropriate to plant redwoods on their property and the process for removing them if they currently exist. -

Vegetation Mapping of Eastman and Hensley Lakes and Environs, Southern Sierra Nevada Foothills, California
Vegetation Mapping of Eastman and Hensley Lakes and Environs, Southern Sierra Nevada Foothills, California By Sara Taylor, Daniel Hastings, Jaime Ratchford, Julie Evens, and Kendra Sikes of the 2707 K Street, Suite 1 Sacramento CA, 95816 2014 ACKNOWLEDGEMENTS To Those Who Generously Provided Support and Guidance: Many groups and individuals assisted us in completing this report and the supporting vegetation map/data. First, we expressly thank an anonymous donor who provided financial support in 2010 for this project’s fieldwork and mapping in the southern foothills of the Sierra Nevada. We also are thankful of the generous support from California Department of Fish and Wildlife (CDFW, previously Department of Fish and Game) in funding 2008 field survey work in the region. We are indebted to the following additional staff and volunteers of the California Native Plant Society who provided us with field surveying, mission planning, technical GIS, and other input to accomplish this project: Jennifer Buck, Andra Forney, Andrew Georgeades, Brett Hall, Betsy Harbert, Kate Huxster, Theresa Johnson, Claire Muerdter, Eric Peterson, Stu Richardson, Lisa Stelzner, and Aaron Wentzel. To Those Who Provided Land Access: Angela Bradley, Ranger, Eastman Lake, U.S. Army Corps of Engineers Bridget Fithian, Mariposa Program Manager, Sierra Foothill Conservancy Chuck Peck, Founder, Sierra Foothill Conservancy Diana Singleton, private landowner Diane Bohna, private landowner Duane Furman, private landowner Jeannette Tuitele-Lewis, Executive Director, Sierra Foothill Conservancy Kristen Boysen, Conservation Project Manager, Sierra Foothill Conservancy Park staff at Hensley Lake, U.S. Army Corps of Engineers i This page has been intentionally left blank. ii TABLE OF CONTENTS Section Page I. -

Reference Plant List
APPENDIX J NATIVE & INVASIVE PLANT LIST The following tables capture the referenced plants, native and invasive species, found throughout this document. The Wildlife Action Plan Team elected to only use common names for plants to improve the readability, particular for the general reader. However, common names can create confusion for a variety of reasons. Common names can change from region-to-region; one common name can refer to more than one species; and common names have a way of changing over time. For example, there are two widespread species of greasewood in Nevada, and numerous species of sagebrush. In everyday conversation generic common names usually work well. But if you are considering management activities, landscape restoration or the habitat needs of a particular wildlife species, the need to differentiate between plant species and even subspecies suddenly takes on critical importance. This appendix provides the reader with a cross reference between the common plant names used in this document’s text, and the scientific names that link common names to the precise species to which writers referenced. With regards to invasive plants, all species listed under the Nevada Revised Statute 555 (NRS 555) as a “Noxious Weed” will be notated, within the larger table, as such. A noxious weed is a plant that has been designated by the state as a “species of plant which is, or is likely to be, detrimental or destructive and difficult to control or eradicate” (NRS 555.05). To assist the reader, we also included a separate table detailing the noxious weeds, category level (A, B, or C), and the typical habitats that these species invade. -

Analysis of California GAP Data: Utility and Limitations of California GAP Data for Regional Conservation Planning Within the C
Analysis of California GAP Data: Utility and Limitations of California GAP Data for Regional Conservation Planning within the Central Valley Flood Protection Program Systemwide Planning Area Prepared for: California Department of Water Resources and AECOM Prepared by: Geographical Information Center California State University, Chico 35 Main Street Chico, California 95929-0327 (530) 898-4372 www.gic.csuchico.edu Contents I. Introduction ................................................................................................................................... 1 II. Data .................................................................................................................................................. 3 a. GAP Analysis Program Land Cover Data ................................................................................ 3 b. Comparative Data ......................................................................................................................... 4 i. Sacramento River Region 2007 Sacramento River Riparian Mapping .................... 4 ii. Feather River Regional Mapping project ..................................................................... 4 iii. Delta Region Land Use Map ........................................................................................... 5 c. Aerial Imagery ............................................................................................................................... 5 III. Methods ......................................................................................................................................... -
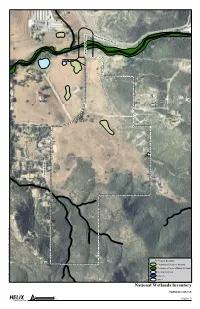
Full Biological Resources Report Outline
e H v ar i mo r n y G D r ove b Roa u d l C y r t n u o C Study Area e v i r D y e r d r o C F K - 4 1 / 2 2 / 7 0 1 0 - V O K d x m . I W N _ 5 g i F \ D J \ O I B \ p a M \ E S e v o r G y n Property Boundary o m r a H _ Freshwater Emergent Wetland 1 0 - V O Freshwater Forested/Shrub Wetland K \ V O K Freshwater Pond \ K \ S T Riverine C E J O R Other P \ : I National Wetlands Inventory HARMONY GROVE 0 350 N Feet Figure 5 e v i r D b k e e u r l C d o C i d o n y s c r E t n u o C Study Area e v i r D y e r d r o C F K - 4 1 / 2 2 / 7 0 Property Boundary 1 0 - V Mule Fat Scrub O K d x Southern Willow Riparian Forest m . n o i t a t Coast Live Oak Woodland e g e V _ 6 Diegan Coastal Sage Scrub g i F \ D J \ Diegan Coastal Sage Scrub - Disturbed O I B \ p a Coastal Sage-chaparral Scrub M \ E S e Mafic Southern Mixed Chaparral v o r G y n Granitic Southern Mixed Chaparral o m r a H _ Non-native Grassland 1 0 - V O Eucalyptus Woodland K \ V O K Non-native Vegetation \ K \ S T Disturbed Habitat C E J O R Developed P \ : I Vegetation HARMONY GROVE 0 350 N Feet Figure 6 the annual, introduced species that comprise the majority of species and biomass within the non-native grassland originated from the Mediterranean region, an area with a long history of agriculture and a climate similar to California. -

Hierarchical List of Natural Communities with Holland Types
September 2010 Global and State Rank CaCode CNDDB Code 1. Mesomorphic Tree Vegetation (Forest and Woodland) 1.C. Temperate Forest 1.C.1. Warm Temperate Forest 1.C.1.c. Madrean Forest and Woodland MG009. California Forest and Woodland Mixed North Slope Forest G4 S4 CTT81500CA Mixed North Slope Cismontane Woodland G3 S3.2 CTT71420CA *Aesculus californica (California buckeye groves) Alliance G3 S3 *75.100.00 *Aesculus californica *75.100.03 *Aesculus californica - Umbellularia californica / Diplacus aurantiacus *75.100.02 *Aesculus californica - Umbellularia californica / Holodiscus discolor *75.100.06 *Aesculus californica / Datisca glomerata *75.100.04 *Aesculus californica / Lupinus albifrons *75.100.05 *Aesculus californica / Toxicodendron diversilobum / moss *75.100.01 *Juglans californica (California walnut groves) Alliance G3 S3 *72.100.00 California Walnut Woodland G2 S2.1 CTT71210CA Walnut Forest G1 S1.1 CTT81600CA *Juglans californica - Quercus agrifolia *72.100.08 *Juglans californica / annual herbaceous *72.100.03 *Juglans californica / Artemisia californica / Leymus condensatus *72.100.04 *Juglans californica / Ceanothus spinosus *72.100.05 *Juglans californica / Heteromeles arbutifolia *72.100.06 *Juglans californica / Malosma laurina *72.100.07 *Lyonothamnus floribundus (Catalina ironwood groves) Special Stands G2 S2 *77.000.00 Island Ironwood Forest G2 S2.1 CTT81700CA Quercus agrifolia (Coast live oak woodland) Alliance G5 S4 (some associations are of 71.060.00 high priority for inventory) Coast Live Oak Woodland G4 S4 CTT71160CA -

Some Notes on California Forest Flora
Proceedings of the Iowa Academy of Science Volume 23 Annual Issue Article 63 1916 Some Notes on California Forest Flora L. H. Pammel Iowa State College Let us know how access to this document benefits ouy Copyright ©1916 Iowa Academy of Science, Inc. Follow this and additional works at: https://scholarworks.uni.edu/pias Recommended Citation Pammel, L. H. (1916) "Some Notes on California Forest Flora," Proceedings of the Iowa Academy of Science, 23(1), 494-517. Available at: https://scholarworks.uni.edu/pias/vol23/iss1/63 This Research is brought to you for free and open access by the Iowa Academy of Science at UNI ScholarWorks. It has been accepted for inclusion in Proceedings of the Iowa Academy of Science by an authorized editor of UNI ScholarWorks. For more information, please contact [email protected]. 494 IOWA ACADEMY OF SCIENCE Pammel: Some Notes on California Forest Flora SOME NOTES ON CALIFORNIA FOREST FLORA. L. H. PAMMEL. Not much that is new can be presented on the forest flora of the state of California. The interesting -forest flora of Cali fornia has been worked over most carefully by a large number of eminent botanists ; of the later contributions we may mention a few of the more recent: namely, the work of Willis Linn Jepson, "The Trees of California"1 and by the same author "The Silva of California,"2 published in 1914, which is the most complete and exhaustive treatise on trees of any given local region; the work of Sudworth, "Forest Trees of the Pa cific Slope;"3 the work of Sargent, "Manual of the Forest Trees of North America Exclusive of Mexico"4 and his monumental work, "The Silva of North America" in fourteen volumes, and Britton's "North American Trees."5 Many botanists have, of course, contributed to our knowledge of the trees of California. -

Sunshine Valley Residence Tree Inventory Moss Beach, Ca
COUNTY OF SAN MATEO - PLANNING AND BUILDING DEPARTMENT ATTACHMENT TREE REPORT For Sunshine Valley Residence LOCATION: Mossbeach, CA Straun Edwards Trees 360 Degrees Certified Arborist #WE5612-A Ph. (408) 898-0625 February 18, 2019 Sunshine Valley Residence APN 037156130 Sunshine Valley Road Moss Beach CA 94038 To whom it may concern: Trees no. 1 & no. 2 are located at the front of the property, along the road side. The Myoporum is fairly insignificant and the Salix is a specimen that has previously failed and is currently growing along the ground. Trees no. 3 & no. 4 (Sequoia and Hesperocyparis) will require removal to allow for construction. Trees no. 5-10 (Myoporum) are old specimens that are located in close proximity to the property boundary lines. All of these trees have fallen and continue to grow along the ground. This growth pattern is typical for the species of older age. It is my professional opinion that none of the Myoporum be classified as significant trees and recommendation that these trees be removed and replaced to create a similar canopy for vegetative screening purposes. Replacement trees could include same species or others such as Melaleuca, Pittosporum, Lauris, Ceanothus, or Salix. Final decision would depend on preference of the homeowner. In regard to the tree protection, at this time I do not deem it necessary to provide tree protection if all trees on site are to be removed. However, should any trees be retained and protection required, I recommend 6 ft. chain link fencing be installed at, or as close to, the drip lines of the canopies throughout all phases of construction.