State of Alaska COVID-19 Vaccination Plan
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

This Is a List of Material Related to the Gates of the Arctic National Park Resident Zoned Communities Which Are Not Found in the University of Alaska System
This is a list of material related to the Gates of the Arctic National Park resident zoned communities which are not found in the University of Alaska system. This list was compiled in 2008 by park service employees. BOOKS: Douglas, Leonard and Vera Douglas. 2000. Kobuk Human-Land Relationships: Life Histories Volume I. Ambler, Kobuk, Shungnak Woods, Wesley and Josephine Woods. 2000. Kobuk Human-Land Relationships: Life Histories Volume 2. Ambler, Kobuk, Shungnak Kunz, Michael L. 1984. Archeology and History in the Upper Kobuk River Drainage: A Report of Phase I of a Cultural Resources Survey and Inventory. Department of the Interior, National Park Service, Gates of the Arctic National Park and Preserve. Kobuk, Ambler, Shungnak Aigner, Jean S. 1981. Cultural resources at Betty, Etivluk, Galbraith-Mosquito, Itkillik, Kinyksukvik, Swayback, and Tukuto Lakes in the Northern foothills of the Brooks Range, Alaska (incomplete citation, possibly incorrect date). Anaktuvuk Pass Aigner, Jean. 1977. A report on the potential archaeological impact of proposed expansion of the Anaktuvuk Pass airstrip facility by the North Slope Borough. Anaktuvuk Pass Alaska Department of Highways, Planning and Research Division. 1973. City of Huslia, Alaska; population 159. Allakaket, Alaska; population 174. Prepared by the State of Alaska, Department of Highways, Planning and Research Division in cooperation with U.S. Department of Transportation, Federal Highway Administration. Allakaket Alexander, Herbert L., Jr. 1968. Archaeology in the Atigun Valley. Expedition 2(1): 35-37. Anaktuvuk Pass Alexander, Herbert L., Jr. 1967. Alaskan survey. Expedition 9(1): 20-29. Anaktuvuk Pass Amsden, Charles W. 1977. Hard times: a case study from northern Alaska, and implications for Arctic prehistory. -

Willows of Interior Alaska
1 Willows of Interior Alaska Dominique M. Collet US Fish and Wildlife Service 2004 2 Willows of Interior Alaska Acknowledgements The development of this willow guide has been made possible thanks to funding from the U.S. Fish and Wildlife Service- Yukon Flats National Wildlife Refuge - order 70181-12-M692. Funding for printing was made available through a collaborative partnership of Natural Resources, U.S. Army Alaska, Department of Defense; Pacific North- west Research Station, U.S. Forest Service, Department of Agriculture; National Park Service, and Fairbanks Fish and Wildlife Field Office, U.S. Fish and Wildlife Service, Department of the Interior; and Bonanza Creek Long Term Ecological Research Program, University of Alaska Fairbanks. The data for the distribution maps were provided by George Argus, Al Batten, Garry Davies, Rob deVelice, and Carolyn Parker. Carol Griswold, George Argus, Les Viereck and Delia Person provided much improvement to the manuscript by their careful editing and suggestions. I want to thank Delia Person, of the Yukon Flats National Wildlife Refuge, for initiating and following through with the development and printing of this guide. Most of all, I am especially grateful to Pamela Houston whose support made the writing of this guide possible. Any errors or omissions are solely the responsibility of the author. Disclaimer This publication is designed to provide accurate information on willows from interior Alaska. If expert knowledge is required, services of an experienced botanist should be sought. Contents -

Soil Survey of Greater Fairbanks Area, Alaska
United States In cooperation with the Department Fairbanks Soil and Soil Survey of of Agriculture Water Conservation District; Alaska Natural Department of Natural Greater Fairbanks Resources Resources, Division of Conservation Agriculture, Division of Service Forestry, Division of Area, Alaska Geological and Geophysical Surveys, and Division of Land; Fairbanks North Star Borough; Tanana Chiefs Conference, Inc.; City of Fairbanks; U.S. Army Corps of Engineers, Chena Lakes Flood Control Project; Alaska Cooperative Extension; University of Alaska Fairbanks, Agricultural and Forestry Experiment Station 3 How To Use This Soil Survey Detailed Soil Maps The detailed soil maps can be useful in planning the use and management of small areas. To find information about your area of interest, locate that area on the Index to Map Sheets. Note the number of the map sheet and turn to that sheet. Locate your area of interest on the map sheet. Note the map unit symbols that are in that area. Turn to the Contents, which lists the map units by symbol and name and shows the page where each map unit is described. The Contents shows which table has data on a specific land use for each detailed soil map unit. Also see the Contents for sections of this publication that may address your specific needs. 4 This soil survey is a publication of the National Cooperative Soil Survey, a joint effort of the United States Department of Agriculture and other Federal agencies, State agencies including the Agricultural and Forestry Experiment Station, and local agencies. The Natural Resources Conservation Service has leadership for the Federal part of the National Cooperative Soil Survey. -

Summary of COVID-19 Vaccine Breakthrough Cases — Alaska, February 1 Through June 30, 2021
Department of Health and Social Services Division of Public Health Editors: Adam Crum, MSPH, Commissioner Heidi Hedberg, Director Joe McLaughlin, MD, MPH Anne Zink, MD, Chief Medical Officer Louisa Castrodale, DVM, MPH 3601 C Street, Suite 540 Local (907) 269-8000 Anchorage, Alaska 99503 http://dhss.alaska.gov/dph/Epi 24 Hour Emergency (800) 478-0084 Bulletin No. 11 July 15, 2021 Summary of COVID-19 Vaccine Breakthrough Cases — Alaska, February 1 through June 30, 2021 Background • 339 (52%) were symptomatic, 253 (38%) were asymptomatic, COVID-19 vaccines remain our best defense against the spread of 64 (10%) were unknown the SARS-CoV-2 virus. The Pfizer-BioNTech, Moderna, and • 95 (38%) of the 253 asymptomatic persons chose a test-based Janssen COVID-19 vaccines are all highly effective at preventing strategy to shorten their isolation by testing negative twice ≥24 hospitalization and death. No vaccines are 100% effective;1,2 hours apart therefore, cases among a small percentage of vaccinated people • 50 (8%) infections were in persons with a history of a previous are expected and are classified as vaccine breakthrough (VB) positive SARS-CoV-2 test >90 days prior to the most recent 3 cases. This Bulletin provides an update of VB cases in Alaska. test; 29 were asymptomatic, 24 had 1 underlying medical All data are preliminary, congruent with public data display as of condition, and 11 were healthcare workers July 14, 2021, and are subject to change. • 62 (9%) were in persons who worked or resided in a long-term Methods care facility Data were summarized for Alaska residents classified as • 200 (59%) of the 338 samples submitted were successfully confirmed or probable cases of SARS-CoV-2 infection from sequenced; 73 (37%) were a variant of concern (VOC; 54 were February 1 through June 30, 2021 who met the definition for a VB Alpha, 2 were Beta, 15 were Delta, and 2 were Gamma). -

COVID-19 Vaccination Programme: Information for Healthcare Practitioners
COVID-19 vaccination programme Information for healthcare practitioners Republished 6 August 2021 Version 3.10 1 COVID-19 vaccination programme: Information for healthcare practitioners Document information This document was originally published provisionally, ahead of authorisation of any COVID-19 vaccine in the UK, to provide information to those involved in the COVID-19 national vaccination programme before it began in December 2020. Following authorisation for temporary supply by the UK Department of Health and Social Care and the Medicines and Healthcare products Regulatory Agency being given to the COVID-19 Vaccine Pfizer BioNTech on 2 December 2020, the COVID-19 Vaccine AstraZeneca on 30 December 2020 and the COVID-19 Vaccine Moderna on 8 January 2021, this document has been updated to provide specific information about the storage and preparation of these vaccines. Information about any other COVID-19 vaccines which are given regulatory approval will be added when this occurs. The information in this document was correct at time of publication. As COVID-19 is an evolving disease, much is still being learned about both the disease and the vaccines which have been developed to prevent it. For this reason, some information may change. Updates will be made to this document as new information becomes available. Please use the online version to ensure you are accessing the latest version. 2 COVID-19 vaccination programme: Information for healthcare practitioners Document revision information Version Details Date number 1.0 Document created 27 November 2020 2.0 Vaccine specific information about the COVID-19 mRNA 4 Vaccine BNT162b2 (Pfizer BioNTech) added December 2020 2.1 1. -
Storage Best Practices for Frozen Vaccines-Fahrenheit
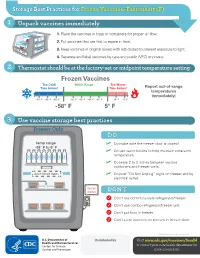
Storage Best Practices for Frozen Vaccines–Fahrenheit (F) 1 Unpack vaccines immediately 1. Place the vaccines in trays or containers for proper air flow. 2. Put vaccines that are first to expire in front. HEP A - VFC 3. Keep vaccines in original boxes with lids closed to prevent exposure to light. 4. Separate and label vaccines by type and public (VFC) or private. 2 Thermostat should be at the factory-set or midpoint temperature setting Frozen Vaccines Too Cold! Within Range Too Warm! Take Action! Take Action! Report out-of-range temperatures immediately! -70° F -65° F -60° F -50° F -45° F -40° F -35° F 10° F 15° F -58° F 5° F 3 Use vaccine storage best practices Freezer Only DO temp range ✓ Do make sure the freezer door is closed! -58° F to 5° F ✓ Do use water bottles to help maintain consistent temperature. ✓ Do leave 2 to 3 inches between vaccine containers and freezer walls. don’t block vents ✓ Do post “Do Not Unplug” signs on freezer and by electrical outlet. do not unplug DON’T Don’t use dormitory-style refrigerator/freezer. Don’t use combo refrigerator/freezer unit. Don’t put food in freezer. Don’t store vaccines on shelves in freezer door. CS243541-D Revision December 2020 Distributed by Visit www.cdc.gov/vaccines/SandH or contact your state health department for more information. Test Your Knowledge 1 Which of the following units is the best for storing frozen vaccines? Freezer Freezer Freezer Freezer A. Full-size B. Full-size C. Stand-alone D. -

CDC Vaccine Storage and Handling Guide
Table of Contents General Information Vaccine Storage and Handling Best Practices 5 Selected Biologicals Diphtheria Toxoid-, Tetanus Toxoid- and acellular Pertussis-Containing Vaccines DTaP: DAPTACEL, Infanrix, Tripedia 11 DTaP-IPV: KINRIX 11 DTaP-HepB-IPV: Pediarix 11 DTaP-IPV/Hib: Pentacel 11 Haemophilus influenzae type b-Containing Vaccines Hib: ActHIB, Hiberix, PedvaxHIB 15 Hib-HepB: Comvax 15 DTaP-IPV/Hib: Pentacel 11 Hepatitis-Containing Vaccines HepA: Havrix, VAQTA 19 HepB: Engerix-B, Recombivax HB 19 HepA-HepB: Twinrix 19 Vaccine Storage and Handling Guide and Storage Vaccine National Center for Immunization and Respiratory Disease DTaP-HepB-IPV: Pediarix 11 Hib-HepB: Comvax 15 Human Papillomavirus Vaccines HPV2: Cervarix 23 HPV4: Gardasil 23 Vaccine Storage and Handling Guide —————————————————————————————— Page 2 Table of Contents Influenza Vaccines LAIV: FluMist 27 TIV: Afluria, Fluarix, FluLaval, Fluvirin, Fluzone, Fluzone High-Dose, Fluzone Intradermal 29 Measles-, Mumps- and Rubella-Containing Vaccine MMR: M-M-RII 33 MMRV: ProQuad 69 Meningococcal Vaccines MCV4: Menactra, Menveo 37 MPSV4: Menomune 41 Pneumococcal Vaccines PCV13: Prevnar 13 45 PPSV23: Pneumovax 23 45 Poliovirus-Containing Vaccine IPV: IPOL 49 DTaP-HepB-IPV: Pediarix 11 DTaP-IPV: KINRIX 11 Vaccine Storage and Handling Guide and Storage Vaccine National Center for Immunization and Respiratory Disease DTaP-IPV/Hib: Pentacel 11 Rotavirus Vaccines RV1: ROTARIX 53 RV5: RotaTeq 53 Tetanus Toxoid Vaccine TT: Tetanus Toxoid 57 Vaccine Storage and Handling Guide —————————————————————————————— -

1.15.20 COVID 19 Mayor's Report AHD Final
Date: January 15, 2021 To: Acting Mayor Austin Quinn-Davidson Thru: Heather Harris, Anchorage Health Department Director From: Janet Johnston, Anchorage Health Department Epidemiologist Subject: January 15, 2021, COVID-19 Risk Assessment Update for the Municipality of Anchorage This weekly report shares data available on the State of Alaska and Municipality of Anchorage (MOA) websites for the period of January 7, 2020 – January 13, 2021, with some more recent data. Unless otherwise indicated, this data is for cases reported in the MOA. Key Findings Municipality of Anchorage COVID-19 metric status for the past week: • RED LIGHT for epidemiology • YELLOW LIGHT for health care capacity • YELLOW LIGHT for public health capacity The average number of new cases has remained relatively stable this week. The current 14-day rolling daily average of 35.9 cases per 100,000 population is slightly lower than one week ago (36.83). However, this is still more than 3.5 times the rate of the State of Alaska’s level for high alert. There were 15% fewer new cases this week than last week. The effective transmission rate (Rt) in Anchorage has increased slightly to 0.94 for Anchorage on January 3. Test positivity dropped to 4.6% this week after increasing above 5% last week. Available ICU beds have decreased again to nine beds, down from 13 last week. Epidemiology These metrics consider case counts and COVID-19-related hospitalizations and deaths. Case Count Trends and Deaths Key Findings: This measure remains RED due to the high level of cases at three and a half times the State’s high alert level. -

Anchorage COVID-19 Health Risk Metrics
Date: May 14, 2021 To: Acting Mayor Austin Quinn-Davidson Thru: Heather Harris, Anchorage Health Department Director From: Janet Johnston, Anchorage Health Department Epidemiologist Subject: May 14, 2021, COVID-19 Risk Assessment Update for the Municipality of Anchorage This weekly report shares data available on the State of Alaska and Municipality of Anchorage (MOA) websites for the period May 6, 2021 – May 12, 2021, with some more recent data. Unless otherwise indicated, this data is for cases reported in the MOA. Anchorage COVID-19 Health Risk Metrics The MOA developed the Anchorage COVID-19 Health Risk Metrics tool to communicate the current level of health risks associated with COVID-19 within the Municipality. The tool includes multiple measures, which are referenced throughout this report. Each metric is assigned one of four categories each week, ranging from Very High Risk to Low Risk. Risk categories are based on public health authorities' standards, including the Centers for Disease Control and Prevention (CDC), the World Health Organization (WHO), and the Harvard Global Health Institute. The Overall Anchorage COVID-19 Risk Level has dropped to the low end of the Considerable Risk category. This Overall Risk Level is a consensus metric derived from individual metrics, with appropriate weight given to the most important metrics – Cases and Health Care Capacity. Additional community- level factors that affect risks associated with COVID-19 within the Municipality are also considered. Average daily new case counts have decreased below 10 cases per day per 100,000 residents, moving us into the Considerable Risk Level for this metric. With just over 50% of eligible residents having completed a COVID-19 vaccines series, Anchorage comprises two subpopulations with very different COVID-19 risk profiles. -

COVID-19 Vaccine Storage & Handling
COVID-19 Vaccine Storage & Handling Brooke Zeringue, Adrienne Whitney, & Robert Starszak Louisiana Department of Health Office of Public Health Immunization Program • Everyone (16 years and older) can be vaccinated. Louisiana Order COVID-19 vaccine in LINKS. Second COVID-19 doses are not automatically ordered. You must Vaccination order every dose you need. Guidelines All vaccine doses administered must be entered into LINKS within 24 hours and inputted as administered, NOT historical. COVID-19 Vaccine Delivery Storage & Handling for Moderna COVID-19 Vaccine: Direct from McKesson Vaccine will arrive frozen Larger quantities Thermal Shipper Storage & Handling for Pfizer- BioNTech COVID-19 Vaccine: Direct from Shipped in thermal shipping container Pfizer Vaccine arrives frozen Larger quantities Storage & Handling for Moderna COVID-19 Vaccine: Morris & Dickson Vaccine will arrive thawed Smaller Quantities Johnson & Johnson (Janssen) COVID-19 Vaccine Vaccine Type: • Viral Vector Vaccine Age indication: Overview of • 18 years or older. the Johnson & Dose & Route: Johnson • 0.5 mL; intramuscular COVID-19 Schedule: Vaccine • Single dose Dose Preparation: • 5 doses per vial Johnson & Johnson | STORING VACCINE VIALS Refrigerator 2°C to 8°C (36°F to 46°F) • Store up to the expiration date • DO NOT store frozen • Protect from light • You can find the expiration date at vaxcheck.jnj Johnson & Johnson | ADMINISTERING • Keep refrigerated until thawed (if arrived frozen). Refrigerator • PUNCTURED VIALS: Viable up to 6 hours 2°C to 8°C (36°F to 46°F) • If arrived frozen and needed immediately, thaw for Room 1 to 2 hours. • UNPUNCTURED VIALS: Viable up to 12 hours kept Temperature at 9°C to 25°C (47°F to 77°F) • PUNCTURED VIALS: Viable up to 2 hours Up to 25°C (Up to 77°F) Johnson & Johnson | ADMINISTERING Visually inspect the Before withdrawing each Johnson & Johnson COVID- dose of vaccine, carefully 19 vaccine vials for other mix the contents by Each dose must contain 0.5 particulate matter and/or swirling gently in an upright mL of vaccine. -

What: AVAP Council Meeting Date & Time: February 20, 2019; 11:00 A.M.-12:30 P.M., AKST Where: BP Energy Center, 900 E Benson
2019-02-20 AVAP Council Meeting Page 1 of 86 c/o KidsVax.org® P.O. Box 1885 • Concord, NH 03302-1885 tel 1.855.KidsVax (543.7829) fax 1.855.KidsFax (543.7329) What: AVAP Council Meeting Date & Time: February 20, 2019; 11:00 a.m.-12:30 p.m., AKST Where: BP Energy Center, 900 E Benson Blvd, Anchorage, AK 99508 Public Access: Please register for the AVAP Council Meeting on February 20, 2019, 11:00 am to 12:30 pm EST at: https://attendee.gotowebinar.com/register/3084090928410617089, Webinar ID: 380-757-811 After registering, you will receive a confirmation email containing information about joining the webinar. Audio PIN: Shown after joining the webinar AVAP Agendas are subject to revision up to and including the time of the meeting. Approx. Time Topic/[Anticipated Action] Presented by: 11:00-11:05 a.m. 1. Welcome J. McLaughlin a. Notification of Recording b. Introductions/Finalization of Agenda 11:05-11:10 a.m. 2. Items for Council Consent J. McLaughlin * a. Approval of Sept. 20, 2018 Council Meeting Minutes 11:10- 11:25 a.m. 3. DHSS Updates M. Bobo a. Regulations b. CDC Vaccination Coverage Award c. 2019-2024 CDC Vaccine for Children Cooperative Agreement d. Enrolling Village Clinics into the Immunization Program e. DHSS Staffing 11:25-11:40 a.m. 4. Legislative Update M. Bobo a. SB 037 (review language of bill) i. Original Bill ii. February 13, 2019 Amendment b. Senate Health Committee Materials c. Senate Finance Committee Materials 11:40-11:50 a.m. -

GAO-09-551 Alaska Native Villages
United States Government Accountability Office Report to Congressional Requesters GAO June 2009 ALASKA NATIVE VILLAGES Limited Progress Has Been Made on Relocating Villages Threatened by Flooding and Erosion GAO-09-551 June 2009 Accountability Integrity Reliability ALASKA NATIVE VILLAGES Highlights Limited Progress Has Been Made on Relocating Highlights of GAO-09-551, a report to Villages Threatened by Flooding and Erosion congressional requesters Why GAO Did This Study What GAO Found In December 2003, GAO reported While the flooding and erosion threats to Alaska Native villages have not been that most of Alaska’s more than completely assessed, since 2003, federal, state, and village officials have 200 Native villages were affected to identified 31 villages that face imminent threats. The U.S. Army Corps of some degree by flooding and Engineers’ (Corps) March 2009 Alaska Baseline Erosion Assessment erosion (GAO-04-142). Since 2003, identified many villages threatened by erosion, but did not assess flooding state officials have identified the growing impacts of climate change, impacts. At least 12 of the 31 threatened villages have decided to relocate—in increasing the urgency of federal part or entirely—or to explore relocation options. and state efforts to identify imminently threatened villages and Federal programs to assist threatened villages prepare for and recover from assess their relocation options. disasters and to protect and relocate them are limited and unavailable to some GAO was asked to report on (1) the villages. The Federal Emergency Management Agency has several disaster flooding and erosion threats that preparedness and recovery programs, but villages often fail to qualify for Alaska Native villages currently them, generally because they may lack approved disaster mitigation plans or face, (2) the federal programs that have not been declared federal disaster areas.