ABSTRACT CHADWICK, ELLE. the Role Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Health Risk Assessment for the Introduction of Eastern Wild Turkeys (Meleagris Gallopavo Silvestris) Into Nova Scotia
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Canadian Cooperative Wildlife Health Centre: Wildlife Damage Management, Internet Center Newsletters & Publications for April 2004 Health risk assessment for the introduction of Eastern wild turkeys (Meleagris gallopavo silvestris) into Nova Scotia A.S. Neimanis F.A. Leighton Follow this and additional works at: https://digitalcommons.unl.edu/icwdmccwhcnews Part of the Environmental Sciences Commons Neimanis, A.S. and Leighton, F.A., "Health risk assessment for the introduction of Eastern wild turkeys (Meleagris gallopavo silvestris) into Nova Scotia" (2004). Canadian Cooperative Wildlife Health Centre: Newsletters & Publications. 48. https://digitalcommons.unl.edu/icwdmccwhcnews/48 This Article is brought to you for free and open access by the Wildlife Damage Management, Internet Center for at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Canadian Cooperative Wildlife Health Centre: Newsletters & Publications by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Health risk assessment for the introduction of Eastern wild turkeys (Meleagris gallopavo silvestris) into Nova Scotia A.S. Neimanis and F.A. Leighton 30 April 2004 Canadian Cooperative Wildlife Health Centre Department of Veterinary Pathology Western College of Veterinary Medicine 52 Campus Dr. University of Saskatchewan Saskatoon, SK Canada S7N 5B4 Tel: 306-966-7281 Fax: 306-966-7439 [email protected] [email protected] 1 SUMMARY This health risk assessment evaluates potential health risks associated with a proposed introduction of wild turkeys to the Annapolis Valley of Nova Scotia. The preferred source for the turkeys would be the Province of Ontario, but alternative sources include the northeastern United States from Minnesota eastward and Tennessee northward. -
2002 FSIS National Residue Program, Section 4
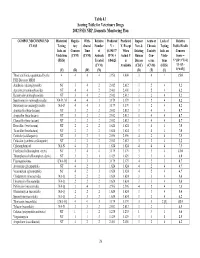
Table 4.1 Scoring Table for Veterinary Drugs 2002 FSIS NRP, Domestic Monitoring Plan COMPOUND/COMPOUND Historical Regula- With- Relative Predicted Predicted Impact Acute or Lack of Relative CLASS Testing tory drawal Number V = V, Except New & Chronic Testing Public Health Info. on Concern Time of (0.19437* When Existing Toxicity Info. on Concern Violations (CVM) (CVM) Animals R*N) + Actual V Human Con- Viola- Score = (FSIS) Treated 0.84625 is Disease cerns tions V*[(D+3*T)/4] (CVM) Available (CDC) (CVM) (FSIS) *{1+[(L- (V) (R) (W) (N) (D) (T) (L) 1)*0.05]} Those antibiotics quantitated by the 4 4 4 4 3.956 4.000 3 4 1 15.0 FSIS Bioassay MRM Amikacin (aminoglycoside) NT 3 4 2 2.012 2.012 3 2 4 5.2 Apramycin (aminoglycoside) NT 4 4 2 2.401 2.401 3 2 4 6.2 Kanamycin (aminoglycoside) NT 3 4 2 2.012 2.012 3 2 4 5.2 Spectinomycin (aminoglycoside) NA-D, M 4 4 3 3.179 3.179 3 2 4 8.2 Streptomycin (aminoglycoside) NA-D 4 4 3 3.179 3.179 3 2 4 8.2 Amoxicillin (beta-lactam) NT 3 2 2 2.012 2.012 3 4 4 8.7 Ampicillin (beta-lactam) NT 3 2 2 2.012 2.012 3 4 4 8.7 Cloxacillin (beta-lactam) NT 3 2 2 2.012 2.012 3 4 4 8.7 Hetacillin (beta-lactam) NT 2 2 2 1.624 1.624 3 4 4 7.0 Ticarcillin (beta-lactam) NT 2 2 2 1.624 1.624 3 4 4 7.0 Ceftiofur (cefalosporin) NT 3 2 3 2.596 2.596 4 2 4 7.5 Cefazolin (synthetic cefalosporin) NT 3 2 2 2.012 2.012 3 2 4 5.2 Chloramphenicol NA-N 4 2 1 1.624 1.624 4 4 4 7.5 Florfenicol (chloramphen. -

Multigene Eukaryote Phylogeny Reveals the Likely Protozoan Ancestors of Opis- Thokonts (Animals, Fungi, Choanozoans) and Amoebozoa
Accepted Manuscript Multigene eukaryote phylogeny reveals the likely protozoan ancestors of opis- thokonts (animals, fungi, choanozoans) and Amoebozoa Thomas Cavalier-Smith, Ema E. Chao, Elizabeth A. Snell, Cédric Berney, Anna Maria Fiore-Donno, Rhodri Lewis PII: S1055-7903(14)00279-6 DOI: http://dx.doi.org/10.1016/j.ympev.2014.08.012 Reference: YMPEV 4996 To appear in: Molecular Phylogenetics and Evolution Received Date: 24 January 2014 Revised Date: 2 August 2014 Accepted Date: 11 August 2014 Please cite this article as: Cavalier-Smith, T., Chao, E.E., Snell, E.A., Berney, C., Fiore-Donno, A.M., Lewis, R., Multigene eukaryote phylogeny reveals the likely protozoan ancestors of opisthokonts (animals, fungi, choanozoans) and Amoebozoa, Molecular Phylogenetics and Evolution (2014), doi: http://dx.doi.org/10.1016/ j.ympev.2014.08.012 This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain. 1 1 Multigene eukaryote phylogeny reveals the likely protozoan ancestors of opisthokonts 2 (animals, fungi, choanozoans) and Amoebozoa 3 4 Thomas Cavalier-Smith1, Ema E. Chao1, Elizabeth A. Snell1, Cédric Berney1,2, Anna Maria 5 Fiore-Donno1,3, and Rhodri Lewis1 6 7 1Department of Zoology, University of Oxford, South Parks Road, Oxford OX1 3PS, UK. -

Epidemiology, Diagnosis and Control of Poultry Parasites
FAO Animal Health Manual No. 4 EPIDEMIOLOGY, DIAGNOSIS AND CONTROL OF POULTRY PARASITES Anders Permin Section for Parasitology Institute of Veterinary Microbiology The Royal Veterinary and Agricultural University Copenhagen, Denmark Jorgen W. Hansen FAO Animal Production and Health Division FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS Rome, 1998 The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. M-27 ISBN 92-5-104215-2 All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying or otherwise, without the prior permission of the copyright owner. Applications for such permission, with a statement of the purpose and extent of the reproduction, should be addressed to the Director, Information Division, Food and Agriculture Organization of the United Nations, Viale delle Terme di Caracalla, 00100 Rome, Italy. C) FAO 1998 PREFACE Poultry products are one of the most important protein sources for man throughout the world and the poultry industry, particularly the commercial production systems have experienced a continuing growth during the last 20-30 years. The traditional extensive rural scavenging systems have not, however seen the same growth and are faced with serious management, nutritional and disease constraints. These include a number of parasites which are widely distributed in developing countries and contributing significantly to the low productivity of backyard flocks. -

Identification of Gene Expression Elements in Histomonas Meleagridis Using Splinkerette Pcr, a Variation of Ligated Adaptor Pcr
IDENTIFICATION OF GENE EXPRESSION ELEMENTS IN HISTOMONAS MELEAGRIDIS USING SPLINKERETTE PCR, A VARIATION OF LIGATED ADAPTOR PCR by ELIZABETH CAROLYN LYNN (Under the Direction of Robert B. Beckstead) ABSTRACT Histomonas meleagridis is the causative agent of blackhead disease in gallinaceous birds, but little genetic information exists for this organism. The complete genome for this protozoan is unsequenced. The only available sequence information is for coding portions of genes. No information is available for expression elements. In this study, we demonstrate that splinkerette PCR procedure, a variation of ligated adaptor PCR, can be used to identify regions upstream and downstream of known coding sequences. Using this technique, we isolated the upstream sequence of 2 beta-tubulin genes. With sequence analysis of their upstream regions, we identified their upstream intergenic regions and 2 different open reading frames. The intergenic region contained putative polyadenylation and cleavage signals and initiator elements. Our research demonstrates that the use of splinkerette PCR is a valuable tool to identify regions of unknown DNA that are 5’ or 3’ to known sequences in parasites whose genomes remain unsequenced. The identification of the expression elements of H. meleagridis will provide tools for future studies on its gene expression. INDEX WORDS: Histomonas meleagridis, molecular characterization, beta-tubulin, splinkerette PCR IDENTIFICATION OF GENE EXPRESSION ELEMENTS IN HISTOMONAS MELEAGRIDIS USING SPLINKERETTE PCR, A VARIATION OF LIGATED ADAPTOR PCR by ELIZABETH CAROLYN LYNN AS, Abraham Baldwin Agricultural College, 2007 BSA, University of Georgia, 2009 A Thesis Submitted to the Graduate Faculty of The University of Georgia in Partial Fulfillment of the Requirements for the Degree MASTER OF SCIENCE ATHENS, GEORGIA 2011 © 2011 Elizabeth Lynn All Rights Reserved IDENTIFICATION OF GENE EXPRESSION ELEMENTS IN HISTOMONAS MELEAGRIDIS USING SPLINKERETTE PCR, A VARIATION OF LIGATED ADAPTOR PCR by ELIZABETH CAROLYN LYNN Major Professor: Robert B. -

Molecular Characterization of Histomonas Meleagridis in Clinical
Original Article Molecular characterization of Histomonas meleagridis in clinical samples of chickens from Eastern China Jinjun Xu1,2 Chanbao Qu1,2 Pin Guo1,2 Zhennan Zhuo1,2 Dandan Liu1,2 Jianping Tao1,2* Abstract Histomonas meleagridis (H. meleagridis) is a protozoan parasite that may cause histomoniasis, a disease of special importance to the poultry industry and public health. The molecular characterization of H. meleagridis in China has not been established. The 5.8S and flanking ITS regions were amplified by polymerase chain reaction from 15 liver samples of chickens which were preliminarily diagnosed with H. meleagridis infection by observing clinical symptoms and macroscopic changes in the organs in Eastern China between 2012 and 2013. The obtained sequences were aligned and compared with other known sequences of H. meleagridis and related protozoan species based on ITS1-5.8S rRNA-ITS2 or 5.8S rRNA region alone. Out of the 15 obtained sequences, 8 sequences were identified as H. meleagridis and were grouped into five clades, suggesting the possibility of multiple genotypes within the samples. Among the remaining 7 sequences, 4 sequences were more related to Trichomonas and 3 sequences were more related to Tetratrichomonas, which suggests the possibility of misdiagnosis or coinfection with other protozoans. Therefore, there is obvious genetic diversity of H. meleagridis based on the 5.8S and flanking ITS regions, which suggests the presence of different genotypes in chickens from Eastern China. Keywords: Histomonas meleagridis, internal transcribed spacer sequence, 5.8S rRNA, homology, phylogenetic relationship 1Jiangsu Co-innovation Center for Prevention and Control of Important Animal Infectious Diseases and Zoonoses, Jiangsu Province 225009, P.R. -

Blackhead Disease in Poultry Cecal Worms Carry the Protozoan That Causes This Disease
Integrated Pest Management Blackhead Disease in Poultry Cecal worms carry the protozoan that causes this disease By Dr. Mike Catangui, Ph.D., Entomologist/Parasitologist Manager, MWI Animal Health Technical Services In one of the most unique forms of disease transmissions known to biology, the cecal worm (Heterakis gallinarum) and the protozoan (Histomonas meleagridis) have been interacting with birds (mainly turkeys and broiler breeders) to perpetuate a serious disease called Blackhead (histomoniasis) in poultry. Also involved are earthworms and house flies that can transmit infected cecal worms to the host birds. Histomoniasis eventually results in fatal injuries to the liver of affected turkeys and chickens; the disease is also called enterohepatitis. Importance Blackhead disease of turkey was first documented in [Fig. 1] are parasites of turkeys, chickens and other the United States about 125 years ago in Rhode Island birds; Histomonas meleagridis probably just started as a (Cushman, 1893). It has since become a serious limiting parasite of cecal worms before it evolved into a parasite factor of poultry production in the U.S.; potential mortalities of turkey and other birds. in infected flocks can approach 100 percent in turkeys and 2. The eggs of the cecal worms (containing the histomonad 20 percent in chickens (McDougald, 2005). protozoan) are excreted by the infected bird into the poultry barn litter and other environment outside the Biology host; these infective cecal worm eggs are picked up by The biology of histomoniasis is quite complex as several ground-dwelling organisms such as earthworms, sow- species of organisms can be involved in the transmission, bugs, grasshoppers, and house flies. -

2019-6654 Final Report of an Audit Carried out In
Ref. Ares(2019)7826731 - 19/12/2019 EUROPEAN COMMISSION DIRECTORATE-GENERAL FOR HEALTH AND FOOD SAFETY Health and food audits and analysis DG(SANTE) 2019-6654 FINAL REPORT OF AN AUDIT CARRIED OUT IN BELARUS FROM 13 TO 24 MAY 2019 IN ORDER TO EVALUATE THE CONTROL OF RESIDUES AND CONTAMINANTS IN LIVE ANIMALS AND ANIMAL PRODUCTS INCLUDING CONTROLS ON VETERINARY MEDICINAL PRODUCTS Executive Summary This report describes the outcome of an audit carried out in Belarus from 13 to 24 May 2019 as part of the European Commission’s Directorate-General for Health and Food Safety planned work programme. The objective of the audit was to evaluate the effectiveness of official controls on residues and contaminants in live animals and animal products eligible for export to the European Union (EU). The audit assessed the implementation of the residue monitoring plan and also covered the authorisation, distribution and use of veterinary medicinal products, given that these areas have an impact on the monitoring of residues. Attention was also paid to examining the implementation of corrective actions indicated in response to specific recommendations made in the report of the previous residues audit to Belarus. The planning of the residue monitoring largely follows the principles of Directive 96/23/EC and covers for the most part an appropriate range of substances. The plan is nevertheless weakened by the fact that action levels for several substances across all commodities (including those for which listing has been requested) are not aligned with EU maximum residue limits, thus the plan would not be sufficient to demonstrate that commodities eligible for export to the EU would comply with such limits where they are lower that national limits. -

Author's Manuscript (764.7Kb)
1 BROADLY SAMPLED TREE OF EUKARYOTIC LIFE Broadly Sampled Multigene Analyses Yield a Well-resolved Eukaryotic Tree of Life Laura Wegener Parfrey1†, Jessica Grant2†, Yonas I. Tekle2,6, Erica Lasek-Nesselquist3,4, Hilary G. Morrison3, Mitchell L. Sogin3, David J. Patterson5, Laura A. Katz1,2,* 1Program in Organismic and Evolutionary Biology, University of Massachusetts, 611 North Pleasant Street, Amherst, Massachusetts 01003, USA 2Department of Biological Sciences, Smith College, 44 College Lane, Northampton, Massachusetts 01063, USA 3Bay Paul Center for Comparative Molecular Biology and Evolution, Marine Biological Laboratory, 7 MBL Street, Woods Hole, Massachusetts 02543, USA 4Department of Ecology and Evolutionary Biology, Brown University, 80 Waterman Street, Providence, Rhode Island 02912, USA 5Biodiversity Informatics Group, Marine Biological Laboratory, 7 MBL Street, Woods Hole, Massachusetts 02543, USA 6Current address: Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, Connecticut 06520, USA †These authors contributed equally *Corresponding author: L.A.K - [email protected] Phone: 413-585-3825, Fax: 413-585-3786 Keywords: Microbial eukaryotes, supergroups, taxon sampling, Rhizaria, systematic error, Excavata 2 An accurate reconstruction of the eukaryotic tree of life is essential to identify the innovations underlying the diversity of microbial and macroscopic (e.g. plants and animals) eukaryotes. Previous work has divided eukaryotic diversity into a small number of high-level ‘supergroups’, many of which receive strong support in phylogenomic analyses. However, the abundance of data in phylogenomic analyses can lead to highly supported but incorrect relationships due to systematic phylogenetic error. Further, the paucity of major eukaryotic lineages (19 or fewer) included in these genomic studies may exaggerate systematic error and reduces power to evaluate hypotheses. -

Colin G. Scanes
Curriculum Vitae COLIN G. SCANES Business Address Home 393S Lapman Hall, 2839 N.Hackett Avenue, University of Wisconsin, Milwaukee Milwaukee, WI 53211 2310 E. Hartford Avenue, USA Milwaukee, WI 53211, USA Telephone: 414-763-1372 Personal Cell: 414-841-8561 Telephone: 414-229- 3641 E-mail: [email protected] E-mail: [email protected] DEGREES 1969 Hull University (U.K.) B.Sc. (Hons.) First Class in Biological Chemistry & Zoology 1972 University of Wales (U.K.). Ph.D., 1972 1985 Hull University (U.K.) D.Sc. CITIZENSHIP US POSITIONS 1972–1978 Lecturer in Animal Physiology & Nutrition, University of Leeds, U.K. 1978–1995 Rutgers—The State University of New Jersey (1978–1982, Associate Professor; 1982–1987 Professor; 1987–1995, Professor II/Distinguished Professor 1981–1995 Chairman, Department of Animal Sciences. 1994–1995 Director, Center for Animal Damage Control, Rutgers University 1995–2000 Executive Associate Dean/Associate Director College of Agriculture/Agricultural Experiment Station, Iowa State University 1999–2001 Interim Director, Plant Science Institute 1995 –2004 Professor, Departments of Animal Science and Biomedical Science, Iowa State University (with continuing courtesy appointments) 2004 -2007 Vice President for Research, Mississippi State University 2007 - 2011 Vice Chancellor for Research and Economic Development/Dean of the Graduate School, University of Wisconsin, Milwaukee 2007 to date Professor of Biological Science and on-going faculty status at Iowa State University (Collaborator professor) and Mississippi State University (Adjunct professor) AWARDS AND HONORS 1986 Rutgers University Board of Trustees Excellence Award for Research. 1990 Paper cited as Citation Classic in Current Contents. 1991 Poultry Science Association, Merck Award for Achievement. -

The Organic Chemistry of Drug Synthesis
The Organic Chemistry of Drug Synthesis VOLUME 2 DANIEL LEDNICER Mead Johnson and Company Evansville, Indiana LESTER A. MITSCHER The University of Kansas School of Pharmacy Department of Medicinal Chemistry Lawrence, Kansas A WILEY-INTERSCIENCE PUBLICATION JOHN WILEY AND SONS, New York • Chichester • Brisbane • Toronto Copyright © 1980 by John Wiley & Sons, Inc. All rights reserved. Published simultaneously in Canada. Reproduction or translation of any part of this work beyond that permitted by Sections 107 or 108 of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful. Requests for permission or further information should be addressed to the Permissions Department, John Wiley & Sons, Inc. Library of Congress Cataloging in Publication Data: Lednicer, Daniel, 1929- The organic chemistry of drug synthesis. "A Wiley-lnterscience publication." 1. Chemistry, Medical and pharmaceutical. 2. Drugs. 3. Chemistry, Organic. I. Mitscher, Lester A., joint author. II. Title. RS421 .L423 615M 91 76-28387 ISBN 0-471-04392-3 Printed in the United States of America 10 987654321 It is our pleasure again to dedicate a book to our helpmeets: Beryle and Betty. "Has it ever occurred to you that medicinal chemists are just like compulsive gamblers: the next compound will be the real winner." R. L. Clark at the 16th National Medicinal Chemistry Symposium, June, 1978. vii Preface The reception accorded "Organic Chemistry of Drug Synthesis11 seems to us to indicate widespread interest in the organic chemistry involved in the search for new pharmaceutical agents. We are only too aware of the fact that the book deals with a limited segment of the field; the earlier volume cannot be considered either comprehensive or completely up to date. -

United States Patent (19) 11) Patent Number: 5,001,115 Sloan (45) Date of Patent: Mar
United States Patent (19) 11) Patent Number: 5,001,115 Sloan (45) Date of Patent: Mar. 19, 1991 54 PRODRUGS OF BIOLOGICALLY ACTIVE Primary Examiner-Mukund J. Shah HYDROXYAROMATIC COMPOUNDS Assistant Examiner-E. L. Ward Attorney, Agent, or Firm-Kerkam, Stowell, Kondracki (75 Inventor: Kenneth B. Sloan, Gainesville, Fla. & Clarke 73 Assignee: University of Florida, Gainesville, Fla, (57) ABSTRACT (21) Appl. No.: 352,919 Prodrugs of bio-active hydroxyaromatic drugs having 22 Filed: May 17, 1989 the structural formula: A pharmaceutically acceptable prodrug of a biologi (51] Int. Cl. ............... A61K 31/70; A61K 31/595; cally active, therapeutically effective hydroxyaromatic A61K 31/535; A61K 31/255 52 U.S. C. ..................................... 514/34: 514/289; drug, said prodrug being selected from the group con 514/169; 514/373; 514/222.8; 514/328; sisting of, (A) compounds having the structural for 514/360; 514/603; 514/417; 514/425; 514/518; mula: 546/44; 54.6/176; 546/75; 544/2; 536/64; 548/123; 548/209; 548/256; 548/417 DRUG-O-CR'R''-2) 58 Field of Search ............... 514/169,289, 373, 417, 514/425, 222.8, 328,360, 518, 603, 34; 536/64; wherein: 564/82, 155; 552/626; 546/44, 176, 75; 544/2; DRUG -O- is the hydroxyaromatic O-dehydro 548/123, 209, 256, 477, 595 residue of said drug; 56 References Cited R" and R' may be the same or different and may be H, PUBLICATIONS alkyl, aryl or electron withdrawing groups; 2 is a displaceable leaving group; and Katritzky, et al. J. Chem. Soc.