External Ca2+ Is Predominantly Used for Cytoplasmic and Nuclear Ca2+ Increases in Fertilized Oocytes of the Marine Bivalve Mactra Chinensis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Analysis of Synonymous Codon Usage Patterns in Sixty-Four Different Bivalve Species
Analysis of synonymous codon usage patterns in sixty-four diVerent bivalve species Marco Gerdol1, Gianluca De Moro1, Paola Venier2 and Alberto Pallavicini1 1 Department of Life Sciences, University of Trieste, Trieste, Italy 2 Department of Biology, University of Padova, Padova, Italy ABSTRACT Synonymous codon usage bias (CUB) is a defined as the non-random usage of codons encoding the same amino acid across diVerent genomes. This phenomenon is common to all organisms and the real weight of the many factors involved in its shaping still remains to be fully determined. So far, relatively little attention has been put in the analysis of CUB in bivalve mollusks due to the limited genomic data available. Taking advantage of the massive sequence data generated from next generation sequencing projects, we explored codon preferences in 64 diVerent species pertaining to the six major evolutionary lineages in Bivalvia. We detected remarkable diVerences across species, which are only partially dependent on phylogeny. While the intensity of CUB is mild in most organisms, a heterogeneous group of species (including Arcida and Mytilida, among the others) display higher bias and a strong preference for AT-ending codons. We show that the relative strength and direction of mutational bias, selection for translational eYciency and for translational accuracy contribute to the establishment of synonymous codon usage in bivalves. Although many aspects underlying bivalve CUB still remain obscure, we provide for the first time an overview of this phenomenon -
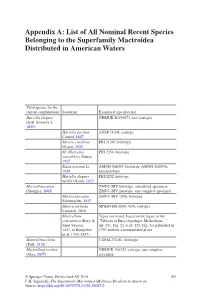
List of All Nominal Recent Species Belonging to the Superfamily Mactroidea Distributed in American Waters
Appendix A: List of All Nominal Recent Species Belonging to the Superfamily Mactroidea Distributed in American Waters Valid species (in the current combination) Synonym Examined type material Harvella elegans NHMUK 20190673, two syntypes (G.B. Sowerby I, 1825) Harvella pacifica ANSP 51308, syntype Conrad, 1867 Mactra estrellana PRI 21265, holotype Olsson, 1922 M. (Harvella) PRI 2354, holotype sanctiblasii Maury, 1925 Raeta maxima Li, AMNH 268093, lectotype; AMNH 268093a, 1930 paralectotype Harvella elegans PRI 2252, holotype tucilla Olsson, 1932 Mactrellona alata ZMUC-BIV, holotype, articulated specimen; (Spengler, 1802) ZMUC-BIV, paratype, one complete specimen Mactra laevigata ZMUC-BIV 1036, holotype Schumacher, 1817 Mactra carinata MNHN-IM-2000-7038, syntypes Lamarck, 1818 Mactrellona Types not found, based on the figure of the concentrica (Bory de “Tableau of Encyclopedique Methodique…” Saint Vincent, (pl. 251, Fig. 2a, b, pl. 252, Fig. 2c) published in 1827, in Bruguière 1797 without a nomenclatorial act et al. 1791–1827) Mactrellona clisia USNM 271481, holotype (Dall, 1915) Mactrellona exoleta NHMUK 196327, syntype, one complete (Gray, 1837) specimen © Springer Nature Switzerland AG 2019 103 J. H. Signorelli, The Superfamily Mactroidea (Mollusca:Bivalvia) in American Waters, https://doi.org/10.1007/978-3-030-29097-9 104 Appendix A: List of All Nominal Recent Species Belonging to the Superfamily… Valid species (in the current combination) Synonym Examined type material Lutraria ventricosa MCZ 169451, holotype; MCZ 169452, paratype; -

Animal Reproduction Science 208 (2019) 106078
Animal Reproduction Science 208 (2019) 106078 Contents lists available at ScienceDirect Animal Reproduction Science journal homepage: www.elsevier.com/locate/anireprosci Ovarian transcriptome analysis of Mactra chinensis provides insights into genes expressed during the intermediate and ripening T stages ⁎ Chongyuan Lin1, Chunyang Guo1, Xiaojing Zhu, Danli Wang, Jilin Xu, Shanliang Xu School of Marine Sciences, Ningbo University, Ningbo 315211, China ARTICLE INFO ABSTRACT Keywords: Enhancing the production of aquatic animals is very important for fishery management and Mactra chinensis aquaculture applications. Ovaries have important functions in producing oocytes and hormones. Transcriptome The Chinese clam (Mactra chinensis) is a nutritious saltwater shellfish. Significant biochemical Ovarian development changes take place during the sexual maturation of M. chinensis; however, the genetic mechan- isms of this process are unclear. Transcriptome sequencing can determine gene expression changes as development occurrs. In the present study, transcriptome sequencing was used to produce a comprehensive transcript dataset for the ovarian development of M. chinensis. The different ovarian developmental stages were determined using hematoxylin-eosin staining. There was identification of 54,172 unigenes at the intermediate stage and 63,081 at the ripening stage, and 80,141 all-unigenes were assembled to determine the molecular mechanism of ovarian de- velopment in M. chinensis. Quantitative real-time PCR for nine mRNAs confirmed the RNA-seq data. Functional annotation of the transcripts indicated there were important pathways in ovarian development, such as those involving the vitellogenin gene. Six pathways associated with ovarian development were identified: estrogen signaling pathway, GnRH signaling pathway, progesterone-mediated oocyte maturation, ovarian steroidogenesis, steroid hormone biosynth- esis, and steroid biosynthesis. -

Fossil Bivalves and the Sclerochronological Reawakening
Paleobiology, 2021, pp. 1–23 DOI: 10.1017/pab.2021.16 Review Fossil bivalves and the sclerochronological reawakening David K. Moss* , Linda C. Ivany, and Douglas S. Jones Abstract.—The field of sclerochronology has long been known to paleobiologists. Yet, despite the central role of growth rate, age, and body size in questions related to macroevolution and evolutionary ecology, these types of studies and the data they produce have received only episodic attention from paleobiologists since the field’s inception in the 1960s. It is time to reconsider their potential. Not only can sclerochrono- logical data help to address long-standing questions in paleobiology, but they can also bring to light new questions that would otherwise have been impossible to address. For example, growth rate and life-span data, the very data afforded by chronological growth increments, are essential to answer questions related not only to heterochrony and hence evolutionary mechanisms, but also to body size and organism ener- getics across the Phanerozoic. While numerous fossil organisms have accretionary skeletons, bivalves offer perhaps one of the most tangible and intriguing pathways forward, because they exhibit clear, typically annual, growth increments and they include some of the longest-lived, non-colonial animals on the planet. In addition to their longevity, modern bivalves also show a latitudinal gradient of increasing life span and decreasing growth rate with latitude that might be related to the latitudinal diversity gradient. Is this a recently developed phenomenon or has it characterized much of the group’s history? When and how did extreme longevity evolve in the Bivalvia? What insights can the growth increments of fossil bivalves provide about hypotheses for energetics through time? In spite of the relative ease with which the tools of sclerochronology can be applied to these questions, paleobiologists have been slow to adopt sclerochrono- logical approaches. -

Chemical Taxonomy of the Hinge-Ligament Proteins of Bivalves According to Their Amino Acid Compositions
Biochem. J. (1987) 242, 505-510 (Printed in Great Britain) 505 Chemical taxonomy of the hinge-ligament proteins of bivalves according to their amino acid compositions Yasuo KIKUCHI* and Nobuo TAMIYA Department of Chemistry, Faculty of Science, Tohoku University Aobayama, Sendai 980, Japan The proteins in the hinge ligaments of molluscan bivalves were subjected to chemotaxonomic studies according to their amino acid compositions. The hinge-ligament protein is a new class of structure proteins, and this is the first attempt to introduce chemical taxonomy into the systematics of bivalves. The hinge-ligament proteins from morphologically close species, namely mactra (superfamily Mactracea) or scallop (family Pectinidae) species, showed high intraspecific homology in their compositions. On the other hand, inconsistent results were obtained with two types of ligament proteins in pearl oyster species (genus Pinctada). The results of our chemotaxonomic analyses were sometimes in good agreement with the morphological classifications and sometimes inconsistent, implying a complicated phylogenetic relationship among the species. INTRODUCTION (1982). The ligaments were removed from the shells and The two shells of molluscan bivalves are connected dried in vacuo. with each other by the organic ligament at the hinge. The Amino acid compositions of the ligament proteins hinge ligament is elastic and functions to open the shells: Small pieces (about 2 mg) of the ligament were heated the ligament is strained when the shells are closed by the in 6 M-HCI (0.3 ml) at 105 °C for 24 h in vacuo. The adductor muscles, and when the muscles relax the amino acids in the hydrolysate were analysed with a JLC spring-like action ofthe elastic ligament opens the valves. -

Shelled Molluscs - Berthou P., Poutiers J.-M., Goulletquer P., Dao J.C
FISHERIES AND AQUACULTURE – Vol. II - Shelled Molluscs - Berthou P., Poutiers J.-M., Goulletquer P., Dao J.C. SHELLED MOLLUSCS Berthou P. Institut Français de Recherche pour l'Exploitation de la Mer, Plouzané, France Poutiers J.-M. Muséum National d’Histoire Naturelle, Paris, France Goulletquer P. Institut Français de Recherche pour l'Exploitation de la Mer, La Tremblade, France Dao J.C. Institut Français de Recherche pour l'Exploitation de la Mer, Plouzané, France Keywords: bivalves, gastropods, fisheries, aquaculture, biology, fishing gears, management Contents 1. Introduction 1.1. Uses of Shellfish: An Overview 1.2. Production 2. Species and Fisheries 2.1. Diversity of Species 2.1.1. Edible Species 2.1.2. Shellfish Species Not Used as Food 2.2. Shelled Molluscs Fisheries 2.2.1. Gastropods 2.2.2. Oysters 2.2.3. Mussels 2.2.4. Scallops 2.2.5. Clams 2.3. Shelled Molluscs Cultivation 2.3.1. Gastropods 2.3.2. Oysters 2.3.3. Mussels 2.3.4. ScallopsUNESCO – EOLSS 2.3.5. Clams 3. Harvesting andSAMPLE Cultivation Techniques CHAPTERS 3.1. Harvesting 3.2. Cultivation techniques 4. Biology 4.1. General Ecology 4.2. Growth 4.3. Reproduction 4.4. Larval Stage in Relation to Dispersal and Stock Abundance 4.5. Migration 5. Stock Assessment and Management Approaches ©Encyclopedia of Life Support Systems (EOLSS) FISHERIES AND AQUACULTURE – Vol. II - Shelled Molluscs - Berthou P., Poutiers J.-M., Goulletquer P., Dao J.C. 5.1. Stock Assessment 5.2. Management Strategies 6. Issues for the Future Bibliography Biographical Sketches Summary Shelled molluscs are comprised of bivalves and gastropods. -

Brachyura: Pinnotheridae), with an Updated List of Symbiont-Host Associations
diversity Review A Review of the Ecomorphology of Pinnotherine Pea Crabs (Brachyura: Pinnotheridae), with an Updated List of Symbiont-Host Associations Werner de Gier 1,2,* and Carola Becker 3 1 Taxonomy and Systematics Group, Naturalis Biodiversity Center, P.O. Box 9517, 2300 RA Leiden, The Netherlands 2 Groningen Institute for Evolutionary Life Sciences, University of Groningen, P.O. Box 11103, 9700 CC Groningen, The Netherlands 3 Vergleichende Zoologie, Institut für Biologie, Humboldt-Universität zu Berlin, Philippstraße 13, Haus 2, 10115 Berlin, Germany; [email protected] * Correspondence: [email protected]; Tel.: +31-7-1751-9600 Received: 14 October 2020; Accepted: 10 November 2020; Published: 16 November 2020 Abstract: Almost all pea crab species in the subfamily Pinnotherinae (Decapoda: Brachyura: Pinnotheridae) are considered obligatory endo- or ectosymbionts, living in a mutualistic or parasitic relationship with a wide variety of invertebrate hosts, including bivalves, gastropods, echinoids, holothurians, and ascidians. While the subfamily is regarded as one of the most morphologically adapted groups of symbiotic crabs, the functionality of these adaptations in relation to their lifestyles has not been reviewed before. Available information on the ecomorphological adaptations of various pinnotherine crab species and their functionality was compiled in order to clarify their ecological diversity. These include the size, shape, and ornamentations of the carapace, the frontal appendages and mouthparts, the cheliped morphology, the ambulatory legs, and the reproductive anatomy and larval characters. The phylogenetic relevance of the adaptations is also reviewed and suggestions for future studies are made. Based on an updated list of all known pinnotherine symbiont–host associations and the available phylogenetic reconstructions, it is concluded that, due to convergent evolution, unrelated species with a similar host interaction might display the same morphological adaptations. -

Population Subdivision of the Surf Clam Mactra Chinensis in the East China
Population subdivision of the surf clam Mactra chinensis in the East China Sea: Changjiang River outflow is not the sole driver Gang Ni1, Qi Li1, Lehai Ni1,2 , Lingfeng Kong1 and Hong Yu1 1 The Key Laboratory of Mariculture, Ministry of Education, Ocean University of China, Qingdao, China 2 Shandong Fisheries Technical Extension Station, Jinan, China ABSTRACT The northwestern Pacific, characterized by unique tectonic and hydrological settings, has greatly intrigued marine phylogeographers. However, current studies mostly focus on the influence of Pleistocene isolation of sea basins in population structure of species in the region, leaving the contribution of other factors (such as freshwater outflow and environmental gradients) largely unexploited. Here we shed light on the question by investigating phylogeography of the surf clam Mactra chinensis in the East China Sea (ECS). Genetic information was acquired from 501 specimens collected from its main distribution in the region, represented by mitochondrial cytochrome oxidase I (COI) and nine polymorphic microsatellite loci. A shallow and star-like phylogeny was revealed for all COI haplotypes, indicating the origin of populations from a single refugium. Although no divergent lineages existed, population subdivision was detected in both data sets. The most striking pattern was the significant diVerentiation between populations north and south of a biogeographic boundary—the Changjiang Estuary, suggesting a barrier eVect of the freshwater outflow to gene flow. For the northern group, substructure was revealed by COI result as one southernmost population was significant Vdi erent from other ones. Clear latitude gradations in allele frequencies were revealed by microsatellite Submitted 31 July 2015 analyses, likely influenced by environmental gradient factors such as temperature. -

Molecular Cytogenetics in Trough Shells (Mactridae, Bivalvia): Divergent GC-Rich Heterochromatin Content
G C A T T A C G G C A T genes Article Molecular Cytogenetics in Trough Shells (Mactridae, Bivalvia): Divergent GC-Rich Heterochromatin Content Daniel García-Souto, Concepción Pérez-García, Jack Kendall and Juan J. Pasantes * Departamento de Bioquímica, Xenética e Inmunoloxía, Universidade de Vigo, E-36310 Vigo, Spain; [email protected] (D.G.-S.); [email protected] (C.P.-G.); [email protected] (J.K.) * Correspondence: [email protected]; Tel.: +34-986-812-577 Academic Editor: Montserrat Corominas Received: 29 June 2016; Accepted: 8 August 2016; Published: 16 August 2016 Abstract: The family Mactridae is composed of a diverse group of marine organisms, commonly known as trough shells or surf clams, which illustrate a global distribution. Although this family includes some of the most fished and cultured bivalve species, their chromosomes are poorly studied. In this work, we analyzed the chromosomes of Spisula solida, Spisula subtruncata and Mactra stultorum by means of fluorochrome staining, C-banding and fluorescent in situ hybridization using 28S ribosomal DNA (rDNA), 5S rDNA, H3 histone gene and telomeric probes. All three trough shells presented 2n = 38 chromosomes but different karyotype compositions. As happens in most bivalves, GC-rich regions were limited to the nucleolus organizing regions in Spisula solida. In contrast, many GC-rich heterochromatic bands were detected in both Spisula subtruncata and Mactra stultorum. Although the three trough shells presented single 5S rDNA and H3 histone gene clusters, their chromosomal locations differed. Regarding major rDNA clusters, while Spisula subtruncata presented a single cluster, both Spisula solida and Mactra stultorum showed two. -

Ruditapes Hybrids
EIDO Escola Internacional de Doutoramento TESE DE DOUTORAMENTO Caracterización citogenética de bivalvos veneroideos y algunos de sus parásitos Cytogenetic characterization of veneroid bivalves and some of their parasites Daniel García Souto 2017 Mención internacional Aquéllos que me busquen, sabrán que he existido. Los demás no tienen necesidad de saberlo. Gustav Mahler AGRADECIMIENTOS Estimado lector anónimo, sea Ud. consciente de que, más allá de la loable función de calzar algún que otro mueble cojo, esta tesis es la consecución, el pináculo quizás, de una etapa de considerable esfuerzo en mi vida. Pero ante todo, sepa que se encuentra ante el trabajo de muchas personas. Esta es, en gran parte la (enésima) tesis de Juanjo, mi director y de Paloma, mi social manager. Ambos han tenido a bien aguantarme un buen rato y, siendo francos, han contribuido tanto (o más) que uno mismo al presente manuscrito. Creo, sinceramente que me habéis ayudado a madurar como investigador y como persona. Espero haber estado a la altura de vuestras expectativas. A ambos muchas gracias. Tras las presentes páginas subyace además el trabajo colaborativo de todo un departamento cuyos integrantes, incluso de forma inconsciente, han contribuido a hacer de mi estancia en el bucólico CUVIlete más cómoda, más agradable y, en definitiva, mejor. Gracias pues a todos los que están y a los que en su momento estuvieron. Esta es también la tesis de los Erasmus-party de la Universidad de Portsmouth (en estricto orden de aparición Neil, Jack, Vesa y Auriel) y de los ocupas de turno del grado en Biología (Jonathan, Pablo, Sonia, Yaiza, Sandra, Susana, Gonzalo y Agustín). -

Germ Cell Differentiation During Spermatogenesis, and Ultrastructural Characteristics of Mature Sperm in Male Phacosoma Japonicus (Bivalvia: Veneridae)
Korean J. Malacol. 27(1): 55-65, 2011 Germ cell Differentiation During Spermatogenesis, and Ultrastructural Characteristics of Mature Sperm in Male Phacosoma japonicus (Bivalvia: Veneridae) Jin Hee Kim1, Ee-Yung Chung2, Moon Sul Choi3, Ki-Young Lee3, IL-Ho Lee4 and Won-Jae Seo2 1Korea Ocean &Fisheries Institute, Busan 608-810, Korea 2Korea Marine Environment & Ecosystem Institute, Dive Korea, Bucheon 420-857, Korea 3Department of Marine Biotechnology, Kunsan National University, Gunsan 573-701, Korea 4Department of Fisheries Science, Graduate School, Kunsan National University, Gunsan 573-701, Korea ABSTRACT Some characteristics of germ cell differntiations during spermiogenesis and mature sperm ultrastructure in male Phacosoma japonicus were investigated by transmission electron microscope observations. The morphology of the spermatozoon of this species has a primitive type and is similar to those of other species in the subclass Heterodonta. Morphologies of the sperm nucleus and the acrosome of this species are the cylindrical type and cap shape, respectively. The spermatozoon is approximately 45-50 μm in length, including a long curved sperm nucleus (about 3.70 μm long with 45° of the angle of the nucleus, an acrosome (about 0.55 μm in length), and tail flagellum (about 42-47 μm). The axoneme of the sperm tail shows a 9+2 structure. As some characteristics of the acrosomal vesicle structures, the basal and lateral parts of basal rings show electron opaque part (region), while the anterior apex part of the acrosomal vesicle shows electron lucent part (region). These characteristics of the acrosomal vesicle were found in the family Veneridae and other several families in the subclass Heterodonta. -

Biological Assessment of Ecologically Important Areas for the Mollusks Taxonomic Group of the Yellow Sea Ecoregion
Biological Assessment Report of the Yellow Sea Ecoregion (2008) Biological Assessment of Ecologically Important Areas for the Mollusks Taxonomic Group of the Yellow Sea Ecoregion China Part Author: LI Baoquan ([email protected]) and LI Xinzheng([email protected]) Position/affiliation: Senior researchers, Institute of Oceanology, Chinese Academy of Sciences Mailing address: 7 Nanhai Road, Qingdao 266071, China Ecological sub-regions Based on existing information, the Yellow Sea Ecoregion (YSE) cannot be further divided into subregions using mollusks as an indicator species. One possibility is that the community structure of mollusks in different parts of the YSE shows no significant differences. Lack of survey data and information may be another explanation. Logic for Mollusk Ecologically Important Areas Selection Twelve Mollusk Ecologically Important Areas (EIA) along the Chinese coast of the Yellow Sea were selected in the YSE biodiversity vision workshop in 2005. The selection is mainly based on: 1) the existence of data and information collected from previous surveys and research; 2) the areas’ proximity to harbor and/or industrial cities, where human activities are bringing the biggest pressure to mollusk habitat. The selected mollusk EIAs are distributed more or less evenly along the Chinese coast of the Yellow Sea; this is a strategy to guarantee these mollusk EIAs can best represent the mollusk habitat diversity of the YSE. The 12 mollusk EIAs are: Lianyungang, Rizhao, Qingdao, Haiyang, Rushan, Weihai, Yantai, Changdao, Tanggu, Qinhuangdao, Huludao, Dalian (see Map 1). Common Criteria for identification of Mollusk EIAs in the YSE The Molluscan Taxonomic Group adopted the following common criteria to identify Ecologically Important Areas for mollusks in YSE (Table 1).