Bryo-Activities: a Review on How Bryophytes Are Contributing to the Arsenal of Natural Bioactive Compounds Against Fungi
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
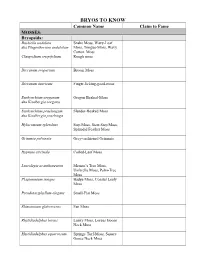
Bryo's to Know Table
BRYOS TO KNOW Common Name Claim to Fame MOSSES: Bryopsida: Buckiella undulata Snake Moss, Wavy-Leaf aka Plagiothecium undulatum Moss, Tongue-Moss, Wavy Cotton, Moss Claopodium crispifolium Rough moss Dicranum scoparium Broom Moss Dicranum tauricum Finger-licking-good-moss Eurhynchium oreganum Oregon Beaked-Moss aka Kindbergia oregana Eurhynchium praelongum Slender-Beaked Moss aka Kindbergia praelonga Hylocomium splendens Step Moss, Stair-Step Moss, Splendid Feather Moss Grimmia pulvinata Grey-cushioned Grimmia Hypnum circinale Coiled-Leaf Moss Leucolepis acanthoneuron Menzie’s Tree Moss, Umbrella Moss, Palm-Tree Moss Plagiomnium insigne Badge Moss, Coastal Leafy Moss Pseudotaxiphyllum elegans Small-Flat Moss Rhizomnium glabrescens Fan Moss Rhytidiadelphus loreus Lanky Moss, Loreus Goose Neck Moss Rhytidiadelphus squarrosum Springy Turf-Moss, Square Goose Neck Moss Rhytidiadelphus triquetrus Electrified Cat-Tail Moss, Goose Necked Moss Rhytidiopsus robusta Robust mountain moss Schistostega pennata Goblin’s Gold, Luminous Moss Polytrichopsida: Atrichum Atrichum Moss , Crane’s Bill Moss (for Atrichum selwynii) Pogonatum contortum Contorted Pogonatum Moss Polytrichum commune Common Hair Cap Moss Polytrichum piliferum Bristly Haircap Moss Andreaeopsida Andreaea nivalis Granite moss, Lantern moss, Snow Rock Moss Sphagnopsida: Sphagnum capillifolium Red Bog Moss, Small Red Peat Moss Sphagnum papillosum Fat Bog Moss, Papillose sphagnum Sphagnum squarrosum Shaggy Sphagnum, Spread- Leaved Peat Moss Takakiopsida: Takakia lepidoziooides Impossible -

Novelties in the Hornwort Flora of Croatia and Southeast Europe
cryptogamie Bryologie 2019 ● 40 ● 22 DIRECTEUR DE LA PUBLICATION : Bruno David, Président du Muséum national d’Histoire naturelle RÉDACTEURS EN CHEF / EDITORS-IN-CHIEF : Denis LAMY ASSISTANTS DE RÉDACTION / ASSISTANT EDITORS : Marianne SALAÜN ([email protected]) MISE EN PAGE / PAGE LAYOUT : Marianne SALAÜN RÉDACTEURS ASSOCIÉS / ASSOCIATE EDITORS Biologie moléculaire et phylogénie / Molecular biology and phylogeny Bernard GOFFINET Department of Ecology and Evolutionary Biology, University of Connecticut (United States) Mousses d’Europe / European mosses Isabel DRAPER Centro de Investigación en Biodiversidad y Cambio Global (CIBC-UAM), Universidad Autónoma de Madrid (Spain) Francisco LARA GARCÍA Centro de Investigación en Biodiversidad y Cambio Global (CIBC-UAM), Universidad Autónoma de Madrid (Spain) Mousses d’Afrique et d’Antarctique / African and Antarctic mosses Rysiek OCHYRA Laboratory of Bryology, Institute of Botany, Polish Academy of Sciences, Krakow (Pologne) Bryophytes d’Asie / Asian bryophytes Rui-Liang ZHU School of Life Science, East China Normal University, Shanghai (China) Bioindication / Biomonitoring Franck-Olivier DENAYER Faculté des Sciences Pharmaceutiques et Biologiques de Lille, Laboratoire de Botanique et de Cryptogamie, Lille (France) Écologie des bryophytes / Ecology of bryophyte Nagore GARCÍA MEDINA Department of Biology (Botany), and Centro de Investigación en Biodiversidad y Cambio Global (CIBC-UAM), Universidad Autónoma de Madrid (Spain) COUVERTURE / COVER : Extraits d’éléments de la Figure 2 / Extracts of -

Seed Plant Models
Review Tansley insight Why we need more non-seed plant models Author for correspondence: Stefan A. Rensing1,2 Stefan A. Rensing 1 2 Tel: +49 6421 28 21940 Faculty of Biology, University of Marburg, Karl-von-Frisch-Str. 8, 35043 Marburg, Germany; BIOSS Biological Signalling Studies, Email: stefan.rensing@biologie. University of Freiburg, Sch€anzlestraße 18, 79104 Freiburg, Germany uni-marburg.de Received: 30 October 2016 Accepted: 18 December 2016 Contents Summary 1 V. What do we need? 4 I. Introduction 1 VI. Conclusions 5 II. Evo-devo: inference of how plants evolved 2 Acknowledgements 5 III. We need more diversity 2 References 5 IV. Genomes are necessary, but not sufficient 3 Summary New Phytologist (2017) Out of a hundred sequenced and published land plant genomes, four are not of flowering plants. doi: 10.1111/nph.14464 This severely skewed taxonomic sampling hinders our comprehension of land plant evolution at large. Moreover, most genetically accessible model species are flowering plants as well. If we are Key words: Charophyta, evolution, fern, to gain a deeper understanding of how plants evolved and still evolve, and which of their hornwort, liverwort, moss, Streptophyta. developmental patterns are ancestral or derived, we need to study a more diverse set of plants. Here, I thus argue that we need to sequence genomes of so far neglected lineages, and that we need to develop more non-seed plant model species. revealed much, the exact branching order and evolution of the I. Introduction nonbilaterian lineages is still disputed (Lanna, 2015). Research on animals has for a long time relied on a number of The first (small) plant genome to be sequenced was of THE traditional model organisms, such as mouse, fruit fly, zebrafish or model plant, the weed Arabidopsis thaliana (c. -

Anthocerotophyta
Glime, J. M. 2017. Anthocerotophyta. Chapt. 2-8. In: Glime, J. M. Bryophyte Ecology. Volume 1. Physiological Ecology. Ebook 2-8-1 sponsored by Michigan Technological University and the International Association of Bryologists. Last updated 5 June 2020 and available at <http://digitalcommons.mtu.edu/bryophyte-ecology/>. CHAPTER 2-8 ANTHOCEROTOPHYTA TABLE OF CONTENTS Anthocerotophyta ......................................................................................................................................... 2-8-2 Summary .................................................................................................................................................... 2-8-10 Acknowledgments ...................................................................................................................................... 2-8-10 Literature Cited .......................................................................................................................................... 2-8-10 2-8-2 Chapter 2-8: Anthocerotophyta CHAPTER 2-8 ANTHOCEROTOPHYTA Figure 1. Notothylas orbicularis thallus with involucres. Photo by Michael Lüth, with permission. Anthocerotophyta These plants, once placed among the bryophytes in the families. The second class is Leiosporocerotopsida, a Anthocerotae, now generally placed in the phylum class with one order, one family, and one genus. The genus Anthocerotophyta (hornworts, Figure 1), seem more Leiosporoceros differs from members of the class distantly related, and genetic evidence may even present -

Checklist of the Liverworts and Hornworts of the Interior Highlands of North America in Arkansas, Illinois, Missouri and Oklahoma
Checklist of the Liverworts and Hornworts of the Interior Highlands of North America In Arkansas, Illinois, Missouri and Oklahoma Stephen L. Timme T. M. Sperry Herbarium ‐ Biology Pittsburg State University Pittsburg, Kansas 66762 and 3 Bowness Lane Bella Vista, AR 72714 [email protected] Paul Redfearn, Jr. 5238 Downey Ave. Independence, MO 64055 Introduction Since the last publication of a checklist of liverworts and hornworts of the Interior Highlands (1997)), many new county and state records have been reported. To make the checklist useful, it was necessary to update it since its last posting. The map of the Interior Highlands of North America that appears in Redfearn (1983) does not include the very southeast corner of Kansas. However, the Springfield Plateau encompasses some 88 square kilometers of this corner of the state and includes limestone and some sandstone and shale outcrops. The vegetation is typical Ozarkian flora, dominated by oak and hickory. This checklist includes liverworts and hornworts collected from Cherokee County, Kansas. Most of what is known for the area is the result of collections by R. McGregor published in 1955. The majority of his collections are deposited in the herbarium at the New York Botanical Garden (NY). This checklist only includes the region defined as the Interior Highlands of North America. This includes the Springfield Plateau, Salem Plateau, St. Francois Mountains, Boston Mountains, Arkansas Valley, Ouachita Mountains and Ozark Hills. It encompasses much of southern Missouri south of the Missouri River, southwest Illinois; most of Arkansas except the Mississippi Lowlands and the Coastal Plain, the extreme southeastern corner of Kansas, and eastern Oklahoma (Fig. -

Introduction to Botany. Lecture 31
Questions and answers Kingdom Vegetabilia: plants Introduction to Botany. Lecture 31 Alexey Shipunov Minot State University November 16, 2011 Shipunov BIOL 154.31 Questions and answers Kingdom Vegetabilia: plants Outline 1 Questions and answers 2 Kingdom Vegetabilia: plants Bryophyta: mosses Shipunov BIOL 154.31 Questions and answers Kingdom Vegetabilia: plants Outline 1 Questions and answers 2 Kingdom Vegetabilia: plants Bryophyta: mosses Shipunov BIOL 154.31 2 Questions and answers Kingdom Vegetabilia: plants Previous final question: the answer 1 Arabidopsis thaliana (L.) Heynh 2 Citrus 3 Piperaceae Where is a genus name? Shipunov BIOL 154.31 Questions and answers Kingdom Vegetabilia: plants Previous final question: the answer 1 Arabidopsis thaliana (L.) Heynh 2 Citrus 3 Piperaceae Where is a genus name? 2 Shipunov BIOL 154.31 Questions and answers Kingdom Vegetabilia: plants Results of Exam 3 (statistical summary) Summary: Min. 1st Qu. Median Mean 3rd Qu. Max. NA’s 43.00 67.00 79.00 78.36 92.00 108.00 5.00 Grades: F D C B max 61 72 82 92 102 Shipunov BIOL 154.31 Questions and answers Kingdom Vegetabilia: plants Results of Exam 3 (the curve) Density estimation for Exam 3 (Biol 154) 61 92 (F) (B) Points Shipunov BIOL 154.31 Questions and answers Bryophyta: mosses Kingdom Vegetabilia: plants Kingdom Vegetabilia: plants Bryophyta: mosses Shipunov BIOL 154.31 Questions and answers Bryophyta: mosses Kingdom Vegetabilia: plants Three main phyla Bryophyta: gametophyte predominance Pteridophyta: sporophyte predominance, no seed Spermatophyta: -

Flora of New Zealand Mosses
FLORA OF NEW ZEALAND MOSSES BRACHYTHECIACEAE A.J. FIFE Fascicle 46 – JUNE 2020 © Landcare Research New Zealand Limited 2020. Unless indicated otherwise for specific items, this copyright work is licensed under the Creative Commons Attribution 4.0 International licence Attribution if redistributing to the public without adaptation: "Source: Manaaki Whenua – Landcare Research" Attribution if making an adaptation or derivative work: "Sourced from Manaaki Whenua – Landcare Research" See Image Information for copyright and licence details for images. CATALOGUING IN PUBLICATION Fife, Allan J. (Allan James), 1951- Flora of New Zealand : mosses. Fascicle 46, Brachytheciaceae / Allan J. Fife. -- Lincoln, N.Z. : Manaaki Whenua Press, 2020. 1 online resource ISBN 978-0-947525-65-1 (pdf) ISBN 978-0-478-34747-0 (set) 1. Mosses -- New Zealand -- Identification. I. Title. II. Manaaki Whenua-Landcare Research New Zealand Ltd. UDC 582.345.16(931) DC 588.20993 DOI: 10.7931/w15y-gz43 This work should be cited as: Fife, A.J. 2020: Brachytheciaceae. In: Smissen, R.; Wilton, A.D. Flora of New Zealand – Mosses. Fascicle 46. Manaaki Whenua Press, Lincoln. http://dx.doi.org/10.7931/w15y-gz43 Date submitted: 9 May 2019 ; Date accepted: 15 Aug 2019 Cover image: Eurhynchium asperipes, habit with capsule, moist. Drawn by Rebecca Wagstaff from A.J. Fife 6828, CHR 449024. Contents Introduction..............................................................................................................................................1 Typification...............................................................................................................................................1 -

Prescribed Fire Decreases Lichen and Bryophyte Biomass and Alters Functional Group Composition in Pacific Northwest Prairies Author(S): Lalita M
Prescribed Fire Decreases Lichen and Bryophyte Biomass and Alters Functional Group Composition in Pacific Northwest Prairies Author(s): Lalita M. Calabria, Kate Petersen, Sarah T. Hamman and Robert J. Smith Source: Northwest Science, 90(4):470-483. Published By: Northwest Scientific Association DOI: http://dx.doi.org/10.3955/046.090.0407 URL: http://www.bioone.org/doi/full/10.3955/046.090.0407 BioOne (www.bioone.org) is a nonprofit, online aggregation of core research in the biological, ecological, and environmental sciences. BioOne provides a sustainable online platform for over 170 journals and books published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Web site, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/page/terms_of_use. Usage of BioOne content is strictly limited to personal, educational, and non-commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. Lalita M. Calabria1, Kate Petersen,The Evergreen State College, 2700 Evergreen Parkway NW, Olympia, Washington 98505 Sarah T. Hamman, The Center for Natural Lands Management, 120 Union Ave SE #215, Olympia, Washington 98501 and Robert J. Smith, Department of Botany and Plant Pathology, 2082 Cordley Hall, Oregon State University, Corvallis, Oregon 97331 Prescribed Fire Decreases Lichen and Bryophyte Biomass and Alters Functional Group Composition in Pacific Northwest Prairies Abstract The reintroduction of fire to Pacific Northwest prairies has been useful for removing non-native shrubs and supporting habitat for fire-adapted plant and animal species. -

Appendix 2: Plant Lists
Appendix 2: Plant Lists Master List and Section Lists Mahlon Dickerson Reservation Botanical Survey and Stewardship Assessment Wild Ridge Plants, LLC 2015 2015 MASTER PLANT LIST MAHLON DICKERSON RESERVATION SCIENTIFIC NAME NATIVENESS S-RANK CC PLANT HABIT # OF SECTIONS Acalypha rhomboidea Native 1 Forb 9 Acer palmatum Invasive 0 Tree 1 Acer pensylvanicum Native 7 Tree 2 Acer platanoides Invasive 0 Tree 4 Acer rubrum Native 3 Tree 27 Acer saccharum Native 5 Tree 24 Achillea millefolium Native 0 Forb 18 Acorus calamus Alien 0 Forb 1 Actaea pachypoda Native 5 Forb 10 Adiantum pedatum Native 7 Fern 7 Ageratina altissima v. altissima Native 3 Forb 23 Agrimonia gryposepala Native 4 Forb 4 Agrostis canina Alien 0 Graminoid 2 Agrostis gigantea Alien 0 Graminoid 8 Agrostis hyemalis Native 2 Graminoid 3 Agrostis perennans Native 5 Graminoid 18 Agrostis stolonifera Invasive 0 Graminoid 3 Ailanthus altissima Invasive 0 Tree 8 Ajuga reptans Invasive 0 Forb 3 Alisma subcordatum Native 3 Forb 3 Alliaria petiolata Invasive 0 Forb 17 Allium tricoccum Native 8 Forb 3 Allium vineale Alien 0 Forb 2 Alnus incana ssp rugosa Native 6 Shrub 5 Alnus serrulata Native 4 Shrub 3 Ambrosia artemisiifolia Native 0 Forb 14 Amelanchier arborea Native 7 Tree 26 Amphicarpaea bracteata Native 4 Vine, herbaceous 18 2015 MASTER PLANT LIST MAHLON DICKERSON RESERVATION SCIENTIFIC NAME NATIVENESS S-RANK CC PLANT HABIT # OF SECTIONS Anagallis arvensis Alien 0 Forb 4 Anaphalis margaritacea Native 2 Forb 3 Andropogon gerardii Native 4 Graminoid 1 Andropogon virginicus Native 2 Graminoid 1 Anemone americana Native 9 Forb 6 Anemone quinquefolia Native 7 Forb 13 Anemone virginiana Native 4 Forb 5 Antennaria neglecta Native 2 Forb 2 Antennaria neodioica ssp. -

Bryophyte Life Cycle
MARCH 2016LEARNING | VELD & FLORA ABOUT BIODIVERSITY Veld & Flora MARCHFACTSHEET: 2016 | VELD MOSS & FLORA 24 25 3. BRYOPHYTE LIFE CYCLE Gametophyte (n) UNDERSTANDING THE GERMINATION MALE ALTERNATION OF GENERATIONS The way that almost all land plants reproduce is by means of two distinct, alternating life forms, a sexual phase that produces and releases gametes or sex cells and allows fertilisation, and a dispersal phase – both of which are adaptations to an essentially waterless environment. The sexual phase is known as the GAMETOPHYTE or haploid spores (n) generation and the dispersal phase is the SPOROPHYTE or diploid (2n) generation. Mature gametophyte plants produce haploid sex cells (egg and sperm) in sex organs (the male antheridia and female archegonia). These sex cells (also called gametes) fuse during fertilisation to form a diploid (2n) zygote which COPING OUT OF WATER grows, by means of mitosis (that results in two daughter cells each having Sperm (n) the same number and kind of chromosomes as the parent cell), into a new released Bryophytes, which include moss, are primitive plants that give us some idea sporophyte plant. The diploid sporophyte produces haploid (n) spores (i.e. from male of how the first plants that ventured onto land coped with their new waterless FEMALE each spore has a single set of chromosomes) by means of the process environment. They share many features with other plants, but differ in some of cell division called meiosis. Meiosis results in four daughter cells each ways – such as the lack of an effective vascular system (specialised tissue for with half the number of chromosomes of the parent cell. -

The Origin of Alternation of Generations in Land Plants
Theoriginof alternation of generations inlandplants: afocuson matrotrophy andhexose transport Linda K.E.Graham and LeeW .Wilcox Department of Botany,University of Wisconsin, 430Lincoln Drive, Madison,WI 53706, USA (lkgraham@facsta¡.wisc .edu ) Alifehistory involving alternation of two developmentally associated, multicellular generations (sporophyteand gametophyte) is anautapomorphy of embryophytes (bryophytes + vascularplants) . Microfossil dataindicate that Mid ^Late Ordovicianland plants possessed such alifecycle, and that the originof alternationof generationspreceded this date.Molecular phylogenetic data unambiguously relate charophyceangreen algae to the ancestryof monophyletic embryophytes, and identify bryophytes as early-divergentland plants. Comparison of reproduction in charophyceans and bryophytes suggests that the followingstages occurredduring evolutionary origin of embryophytic alternation of generations: (i) originof oogamy;(ii) retention ofeggsand zygotes on the parentalthallus; (iii) originof matrotrophy (regulatedtransfer ofnutritional and morphogenetic solutes fromparental cells tothe nextgeneration); (iv)origin of a multicellularsporophyte generation ;and(v) origin of non-£ agellate, walled spores. Oogamy,egg/zygoteretention andmatrotrophy characterize at least some moderncharophyceans, and arepostulated to represent pre-adaptativefeatures inherited byembryophytes from ancestral charophyceans.Matrotrophy is hypothesizedto have preceded originof the multicellularsporophytes of plants,and to represent acritical innovation.Molecular -

Taxonomical and Nomenclatural Notes on the Moss Ceratodon Conicus (Ditrichaceae, Bryophyta) Author(S): Marta Nieto-Lugilde, Olaf Werner & Rosa M
Taxonomical and Nomenclatural Notes on the Moss Ceratodon conicus (Ditrichaceae, Bryophyta) Author(s): Marta Nieto-Lugilde, Olaf Werner & Rosa M. Ros Source: Cryptogamie, Bryologie, 39(2):195-200. Published By: Association des Amis des Cryptogames https://doi.org/10.7872/cryb/v39.iss2.2018.195 URL: http://www.bioone.org/doi/full/10.7872/cryb/v39.iss2.2018.195 BioOne (www.bioone.org) is a nonprofit, online aggregation of core research in the biological, ecological, and environmental sciences. BioOne provides a sustainable online platform for over 170 journals and books published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Web site, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/page/terms_of_use. Usage of BioOne content is strictly limited to personal, educational, and non- commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. Cryptogamie, Bryologie, 2018, 39 (2): 195-200 © 2018 Adac. Tous droits réservés taxonomical and nomenclatural notes on the moss Ceratodon conicus (ditrichaceae, Bryophyta) marta nıeTo-lUGılDe, olaf Werner &rosa m. ros * Departamento de Biologíavegetal, Facultad de Biología, Universidad de murcia, Campus de espinardo, 30100 murcia, spain Abstract – Arevision of the nomenclatural and taxonomical data related to Ceratodon conicus (Hampe ex Müll. Hal.) Lindb. and its synonyms published by Burley &Pritchard (1990) was carried out.