A Review of Landfill Leachate Treatment by Microalgae: Current
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

From the Past to the Future of Landfill Engineering Through Case Histories
Missouri University of Science and Technology Scholars' Mine International Conference on Case Histories in (1998) - Fourth International Conference on Geotechnical Engineering Case Histories in Geotechnical Engineering 08 Mar 1998 - 15 Mar 1998 From the Past to the Future of Landfill Engineering Through Case Histories R. Kerry Rowe University of Western Ontario, London, Ontario, Canada Follow this and additional works at: https://scholarsmine.mst.edu/icchge Part of the Geotechnical Engineering Commons Recommended Citation Rowe, R. Kerry, "From the Past to the Future of Landfill Engineering Through Case Histories" (1998). International Conference on Case Histories in Geotechnical Engineering. 4. https://scholarsmine.mst.edu/icchge/4icchge/4icchge-session00/4 This Article - Conference proceedings is brought to you for free and open access by Scholars' Mine. It has been accepted for inclusion in International Conference on Case Histories in Geotechnical Engineering by an authorized administrator of Scholars' Mine. This work is protected by U. S. Copyright Law. Unauthorized use including reproduction for redistribution requires the permission of the copyright holder. For more information, please contact [email protected]. 145 Proceedings: Fourth International Conference on Case Histories in Geotechnical Engineering~ St. Louis, Missouri, March 9-12, 1998. FROM THE PAST TO THE FUTURE OF LANDFILL ENGINEERING THROUGH CASE HISTORIES R. Kerry Rowe Paper No. SOA-9 Dept. of Civil & Environmental Engineering University of Western Ontario London, Ontario, Canada N6A 5B9 AIISTRACT The advances in landfill engineering are outlined based on a number of case histories illustrating past problems, hydraulic performance of clay liners, diffusive transport through liners, hydraulic containment and clogging of leachate collection systems. -

Compliance Boundary at the Keele Valley Landfill Site
Compliance Boundary at the Keele Valley Landfill Site (City Council on August 1, 2, 3 and 4, 2000, adopted this Clause, without amendment.) The Policy and Finance Committee and the Works Committee jointly recommend the adoption of the following report (June 27, 2000) from the Commissioner of Works and Emergency Services: Purpose: To obtain the approval of City Council to request the Ministry of the Environment (MOE) to move the compliance boundary of the Keele Valley Landfill Site from the edge of the secondary buffer lands south of the City-owned lands northwards to the south end of a modified primary buffer, to take place upon the completion of landfilling operations at Keele Valley. Financial Implications and Impact Statement: If the recommendation is approved, subject to the conditions suggested, there are no financial implications to the City of Toronto. Recommendations: It is recommended that: (1) the City of Toronto request the Ministry of the Environment to amend the Certificate of Approval applicable to the Keele Valley Landfill Site to move the compliance boundary of the landfill from the south end of the secondary buffer lands at Major Mackenzie Drive to the south of the primary buffer lands as redefined as set out in this report; (2) Recommendation No. (1) be subject to the following conditions: (a) York Major Holdings, the owner of the lands comprising the secondary buffer, enter into an agreement with the City of Toronto incorporating the following provisions: (i) any new land use on the lands that are currently part of -

(I) CITY of VAUGHAN COUNCIL MINUTES JUNE 23, 2003 Table Of
CITY OF VAUGHAN COUNCIL MINUTES JUNE 23, 2003 Table of Contents Minute No. Page No. 138. PRESENTATION..........................................................................................................................118 139. VERBAL REPORT WITH RESPECT TO THE SMOG SUMMIT .................................................118 140. CONFIRMATION OF AGENDA....................................................................................................119 141. DISCLOSURE OF INTEREST .....................................................................................................120 142. ADOPTION OR CORRECTION OF MINUTES............................................................................120 143. DETERMINATION OF ITEMS REQUIRING SEPARATE DISCUSSION.....................................120 144. CONSIDERATION OF ITEMS REQUIRING SEPARATE DISCUSSION ....................................121 145. WILLIAM GRANGER GREENWAY – BARTLEY SMITH GREENWAY (Supplementary Report No. 3)......................................................................................................123 146. ZONING BY-LAW AMENDMENT FILE Z.01.008 DRAFT PLAN OF SUBDIVISION FILE 19T-01V02 MATTHEW GABRIELE & MICHELA TONIETTO REPORT #P.2001.20 (Supplementary Report No. 4) .................................................................................125 147. CONSIDERATION OF ITEMS REQUIRING SEPARATE DISCUSSION ....................................130 148. KEELE VALLEY SMALL VEHICLE TRANSFER STATION AND HHW DEPOT (Addendum No. 3) ........................................................................................................................130 -

Long Range Solid Waste Management Plan Environmental Assessment
Long Range Solid Waste Management Plan Environmental Assessment Appendix H – Design and Operations Report August 2007 Long Range Solid Waste Management Plan Environmental Assessment Appendix H – Design and Operations Report - August 2007 TABLE OF CONTENTS Page 1.0 INTRODUCTION .............................................................................................. H-1 1.1 Purpose and Scope ............................................................................... H-1 1.2 Regulatory Requirements ...................................................................... H-1 1.3 Background.......................................................................................... H-10 1.4 Description of the Undertaking............................................................. H-11 2.0 LANDFILL EXPANSION SITE DESCRIPTION.............................................. H-13 2.1 Site Location ........................................................................................ H-13 2.2 Site Boundaries ................................................................................... H-13 2.3 Land Use ............................................................................................. H-13 2.4 Topography.......................................................................................... H-13 2.5 Hydrology............................................................................................. H-14 2.6 Hydrogeology....................................................................................... H-14 2.7 Archaeology........................................................................................ -
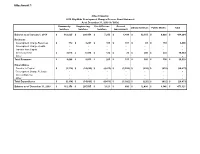
Attachment 1
Attachment 1 City of Vaughan 2019 City-Wide Development Charges Reserve Fund Statement As at December 31, 2019 (in '000s) Community Engineering Fire & Rescue General Library Services Public Works Total Services Services Services Government Balance as of January 1, 2019 $ 162,825 $ 290,454 $ 7,253 $ 1,499 $ 12,365 $ 9,888 $ 484,284 Revenues Development Charge Revenues $ 772 $ 3,297 $ 161 $ 167 $ 81 $ 169 4,646 Development Charge Credits - - - - - - - Transfer from Capital - - - - - - - Interest Earned $ 3,676 $ 6,538 $ 122 $ 26 $ 280 $ 222 10,864 Other - - - - - - - Total Revenues $ 4,448 $ 9,835 $ 283 $ 193 $ 360 $ 390 $ 15,510 Expenditures Transfer to Capital $ (5,114) $ (14,695) $ (4,413) $ (1,092) $ (226) $ (932) (26,473) Development Charge Refunds - - - - - - - Interest Expense - - - - - - - Other - - - - - - - Total Expenditures $ (5,114) $ (14,695) $ (4,413) $ (1,092) $ (226) $ (932) $ (26,473) Balance as of December 31, 2019 $ 162,158 $ 285,595 $ 3,123 $ 600 $ 12,499 $ 9,346 $ 473,321 City of Vaughan 2019 Area Specific Development Charges Reserve Fund Statement As at December 31, 2019 D8 - Rainbow D15 - West D18 - West D19 - East PD D20 - D23 - Dufferin D24 - Ansley Creek Woodbridge Major Mack Rutherford Watermain W. Teston Grove Balance as of January 1, 2019 $ 3,788 $ (244) $ (249) $ 802 $ 2,618 $ 89 $ 224 Revenues Development Charge Revenues 13 37 - - - - - Transfer from Capital - - - - - - - Interest Earned 86 10 (1) 4 59 2 5 Other - - - - - - - Total Revenues $ 99 $ 47 $ (1) $ 4 $ 59 $ 2 $ 5 Expenditures Transfer to Capital - 757 258 (756) - - - Development Charge Refunds - - - - - - - Interest Expense - - - - - - - Other - - - - - - - Total Expenditures $ - $ 757 $ 258 $ (756) $ - $ - $ - Balance as of December 31, 2019 $ 3,887 $ 559 $ 8 $ 50 $ 2,677 $ 91 $ 229 D33 - D25 - Zenway D27 - Black Creek Black Creek Woodbridge Total Fogel Huntington Map 2 Map 3 Ave. -

2 Exchange of Interests in Land Portions of the Keele Valley Landfill
CITY CLERK Clause embodied in Report No. 17 of the Administration Committee, as adopted by the Council of the City of Toronto at its meeting held on December 4, 5 and 6, 2001. 2 Exchange of Interests in Land Portions of the Keele Valley Landfill Site (Vaughan) (City Council on December 4, 5 and 6, 2001, amended this Clause by adding thereto the following: “It is further recommended that the joint report dated November 29, 2001, from the Commissioner of Corporate Services and the Commissioner of Works and Emergency Services, embodying the following recommendations, be adopted: ‘It is recommended that: (1) authority be granted for the City to enter into an agreement with York Major Holdings Inc. (“York Major”) to effect an exchange of interests in land at the Keele Valley Landfill site on the terms outlined in the body of this report; (2) the City Solicitor be authorized to complete the transaction on behalf of the City, including payment of any necessary expenses; (3) Recommendation No. (2)(b)(i) of Clause No. 2 of Joint Policy and Finance and Works Committee Report No. 2, as adopted by Council at its meeting held on August 1, 2, 3 and 4, 2000, be amended to require the City of Vaughan to first enact a temporary Zoning By-law to permit composting at the Avondale Facility to continue until December 31, 2003, instead of until May 31, 2004; and (4) the appropriate City officials be authorized and directed to take necessary action to give effect thereto.’ ”) (City Council on November 6, 7 and 8, 2001, deferred consideration of this Clause to the next regular meeting of City Council scheduled to be held on December 4, 2001.) Toronto City Council 2 Administration Committee December 4, 5 and 6, 2001 Report No. -

North Maple Regional Park Phase 2 Development Update January 2021
Committee of the Whole (1) Report DATE: Tuesday, January 19, 2021 WARD(S): ALL TITLE: NORTH MAPLE REGIONAL PARK PHASE 2 DEVELOPMENT UPDATE JANUARY 2021 FROM: Nick Spensieri, Deputy City Manager, Infrastructure Development ACTION: FOR INFORMATION Purpose To provide an update on Phase 2 park development at North Maple Regional Park (NMRP) including progress by the Technical Advisor consultant team on completion of 30% design and technical studies. Report Highlights The first part of Phase 2 park development involves preparing a large area of undeveloped parkland with major site grading, servicing (water, sanitary, electrical, storm water) and restoration of the pond and wetland areas which are provincially significant. In August 2020 the Technical Advisor consultant team contract was awarded to Stantec Consulting Ltd. and the project was initiated in September 2020 Progress on Phase 2 design and technical studies to date includes completion of initial stakeholder meetings, site investigations, draft design submissions and reports. A procurement process for the Design-Build portion of Phase 2 site preparation, grading and servicing is currently planned and on-schedule to commence in Q2 2021. Recommendations 1. That this report be received for information. Item 7 Page 1 of 6 Background In April 2018, Council endorsed the 900-acre vision for NMRP to create a nationally significant public sports, recreation and cultural venue as a legacy project for Vaughan residents and visitors. In September 2018, Phase 1A park development (artificial turf fields, driveway, parking and pathways) was completed and the park officially opened for use. Phase 1B park development (washrooms, changerooms, shade shelters and event preparations) was completed in 2019 with the first Canada Day event held at NMRP on July 1, 2019 with over 18,000 visitors attending. -

Update – Eastern Power Royalties
CITY OF VAUGHAN EXTRACT FROM COUNCIL MEETING MINUTES OF SEPTEMBER 20, 2016 Item 10, Report No. 9, of the Finance, Administration and Audit Committee, which was adopted without amendment by the Council of the City of Vaughan on September 20, 2016. 10 UPDATE – EASTERN POWER ROYALTIES The Finance, Administration and Audit Committee recommends approval of the recommendation contained in the following report of the Chief Financial Officer/City Treasurer and the Director of Financial Planning and Development Finance, dated September 6, 2016: Recommendation The Chief Financial Officer/City Treasurer and the Director of Financial Planning and Development Finance recommend: 1. That this report be received for information purposes. Contribution to Sustainability The Keele Valley Landfill reserve holds funds from the royalties paid by the City of Toronto in accordance with the agreement between the the two Cities. The royalties Keele Valley Landfill Reserve is a funding source for beautification efforts in the Maple community. Economic Impact The City has received notification from the City of Toronto that Eastern Power Ltd. has ceased power production at the Keele Valley Landfill site as of December 2015, as per the terms of the original contract with the Metropolitan Corporation (City of Toronto). Cessation of operations has resulted in the termination of royalty revenues received by the City of Toronto. In turn, this has resulted in the termination of the receipt of ten percent of these revenues by the City of Vaughan. Communications Plan The report is available publicly on the Agenda, Minutes & Extract page of the City’s website (www.vaughan.ca). Purpose To provide an update to Council on the status of electricity production by Eastern Power Ltd. -

Baseflow and Water Use Assessment – Report on Current Conditions
Don River Watershed Plan Baseflow and Water Use Assessment – Report on Current Conditions 2009 Prepared by: Toronto and Region Conservation Don River Watershed Plan: Baseflow and Water Use Assessment – Report on Current Conditions Table of Contents Table of Contents............................................................................................................................ 2 List of Tables................................................................................................................................... 3 List of Figures.................................................................................................................................. 3 1.0 Introduction............................................................................................................................... 4 2.0 Understanding Groundwater Recharge and Discharge .......................................................... 4 2.1 Don River Watershed Water Budget Modeling .................................................................... 5 2.2 Regional Groundwater Modeling.......................................................................................... 5 2.3 TRCA Role/Interest in Low Flow ........................................................................................... 5 3.0 Data Sources and Methods...................................................................................................... 6 3.1 Baseflow Data Collection ..................................................................................................... -

Solid Waste Management Services Telephone Directory
1 Solid Waste Management Services Solid Waste Management Services Infrastructure and Development Services Solid Waste Management Services is responsible for collecting, transporting, processing, composting and disposing of municipal and some private sector solid waste, including garbage, recyclables, organics, yard waste, electronics and household hazardous waste. The Division manages four collections yards, one maintenance yard, seven transfer stations, six household hazardous waste depots, two organics processing facilities, Green Lane Landfill and 160 former landfills. The Division’s customers include: • Approximately 870,000 homes and non-residential establishments o 462,000 single family residential (includes 11,000 residential units above commercial) o 400,000 multi-residential units o 7,500 non-residential establishments (includes small businesses, charities, institutions and religious organizations) • Schools, City Divisions, Agencies and Corporations • Approximately 10,000 street litter/recycling bins • 10,000 garbage and recycling bins in City parks • 1,000 Special Events per year • Private Industrial, Commercial and Institutional waste accepted at Drop-off Depots and landfill Toronto City Hall People Services Consultant (Acting) 25th Fl. E., 100 Queen St. W Workforce Planning Toronto ON M5H 2N2 Cavelle Landeau ............................. 392-5792 People Services Associate (Acting) Workforce Planning Facsimile ............................................... 392-4754 Lawrence Davidson ........................ 392-7449 Chief -
2 New Artificial Soccer Turfs Could Be on Way for Vaughan
2 new artificial soccer turfs could be on way for Vaughan Feb 11, 2015 | Vote 0 0 2 new artificial soccer turfs could be on way for Vaughan Vaughan Citizen By Adam Martin-Robbins A capacity crowd packed the council chamber at city hall Monday night. And the vast majority of them were players, coaches and parents from the Vaughan Soccer Club who came for one very specific reason. They were there to let the city’s budget committee know they want two artificial turf soccer fields, not just one, built at North Maple Regional Park and they want work to begin on both this year. “Approving the first phase, with the two turf fields and walking trail, is a long awaited start for this community,” Lucille Abate, past president of the York Region Soccer Association, said. “We stand before you this evening, filling your stands, to urge the city to expedite this process as soon as possible. This park will provide the core services for over 70,000 residents who live in north-east Vaughan.” Abate noted the soccer club has agreed to contribute $150,000 toward the project, which expected to cost about $5.4 million and take 15 to 18 months to complete. A representative of Vaughan C.A.R.E.S (Committee of Associations to Restore Environmental Safety) also spoke in favour of the project. City staff, who have been struggling to get the property tax increase down to at least 3 per cent as directed by council, considered going ahead with one field instead of two this year. -

Don River Watershed Plan Beyond Forty Steps
DON RIVER WATERSHED PLAN BEYOND FORTY STEPS 2009 Prepared by: Toronto and Region Conservation © Toronto and Region Conservation 2009 ISBN: 978-0-9811107-4-5 www.trca.on.ca 5 Shoreham Drive Toronto, Ontario M3N 1S4 phone: 416-661-6600 fax: 416-661-6898 DON RIVER WATERSHED PLAN BEYOND FORTY STEPS 2009 Prepared by: Toronto and Region Conservation Acknowledgements This Don River Watershed Plan, prepared under the direction of the Toronto and Region Conservation Authority and the Don Watershed Regeneration Council, represents the combined effort of many partici- pants. Appreciation and thanks are extended to the writer William Glenn; to Mark Schollen for his concept site designs; to the members of the Don Watershed Regeneration Council, as listed in Appendix C; to the Toronto and Region Conservation Authority staff and consultants, as listed in Appendix D; and to the members of the Municipal Technical Advisory Committee, as listed in Appendix E. All photography © Toronto and Region Conservation 2009 unless otherwise specified. The data used to create all maps in this document were compiled from a variety of sources and dates. The TRCA takes no responsibility for errors or omissions in the data and retains the right to make changes and corrections at anytime without notice. For further information about the data on these maps, please contact the TRCA GIS Department at (416) 661-6600. All maps created by: Information Systems/ Information Technology. www.trca.on.ca 5 Shoreham Drive Toronto, Ontario M3N 1S4 Phone: 416-661-6600 Fax: 416-661-6898 Don River Watershed Plan, 2009 ii Executive Summary he Don River Watershed Plan builds on the hard-won gains made to date in protecting, regenerating and taking collec- T tive responsibility for this abused but still beautiful feature of our natural heritage.