LESSON CLUSTER 1 States of Water
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
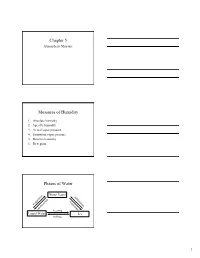
Chapter 5 Measures of Humidity Phases of Water
Chapter 5 Atmospheric Moisture Measures of Humidity 1. Absolute humidity 2. Specific humidity 3. Actual vapor pressure 4. Saturation vapor pressure 5. Relative humidity 6. Dew point Phases of Water Water Vapor n o su ti b ra li o n m p io d a a at ep t v s o io e en s n d it n io o n c freezing Liquid Water Ice melting 1 Coexistence of Water & Vapor • Even below the boiling point, some water molecules leave the liquid (evaporation). • Similarly, some water molecules from the air enter the liquid (condense). • The behavior happens over ice too (sublimation and condensation). Saturation • If we cap the air over the water, then more and more water molecules will enter the air until saturation is reached. • At saturation there is a balance between the number of water molecules leaving the liquid and entering it. • Saturation can occur over ice too. Hydrologic Cycle 2 Air Parcel • Enclose a volume of air in an imaginary thin elastic container, which we will call an air parcel. • It contains oxygen, nitrogen, water vapor, and other molecules in the air. 1. Absolute Humidity Mass of water vapor Absolute humidity = Volume of air The absolute humidity changes with the volume of the parcel, which can change with temperature or pressure. 2. Specific Humidity Mass of water vapor Specific humidity = Total mass of air The specific humidity does not change with parcel volume. 3 Specific Humidity vs. Latitude • The highest specific humidities are observed in the tropics and the lowest values in the polar regions. -

Insar Water Vapor Data Assimilation Into Mesoscale Model
This article has been accepted for inclusion in a future issue of this journal. Content is final as presented, with the exception of pagination. IEEE JOURNAL OF SELECTED TOPICS IN APPLIED EARTH OBSERVATIONS AND REMOTE SENSING 1 InSAR Water Vapor Data Assimilation into Mesoscale Model MM5: Technique and Pilot Study Emanuela Pichelli, Rossella Ferretti, Domenico Cimini, Giulia Panegrossi, Daniele Perissin, Nazzareno Pierdicca, Senior Member, IEEE, Fabio Rocca, and Bjorn Rommen Abstract—In this study, a technique developed to retrieve inte- an extremely important element of the atmosphere because its grated water vapor from interferometric synthetic aperture radar distribution is related to clouds, precipitation formation, and it (InSAR) data is described, and a three-dimensional variational represents a large proportion of the energy budget in the atmo- assimilation experiment of the retrieved precipitable water vapor into the mesoscale weather prediction model MM5 is carried out. sphere. Its representation inside numerical weather prediction The InSAR measurements were available in the framework of the (NWP) models is critical to improve the weather forecast. It is European Space Agency (ESA) project for the “Mitigation of elec- also very challenging because water vapor is involved in pro- tromagnetic transmission errors induced by atmospheric water cesses over a wide range of spatial and temporal scales. An vapor effects” (METAWAVE), whose goal was to analyze and pos- improvement in atmospheric water vapor monitoring that can sibly predict the phase delay induced by atmospheric water vapor on the spaceborne radar signal. The impact of the assimilation on be assimilated in NWP models would improve the forecast the model forecast is investigated in terms of temperature, water accuracy of precipitation and severe weather [1], [3]. -

Chapter 3 Equations of State
Chapter 3 Equations of State The simplest way to derive the Helmholtz function of a fluid is to directly integrate the equation of state with respect to volume (Sadus, 1992a, 1994). An equation of state can be applied to either vapour-liquid or supercritical phenomena without any conceptual difficulties. Therefore, in addition to liquid-liquid and vapour -liquid properties, it is also possible to determine transitions between these phenomena from the same inputs. All of the physical properties of the fluid except ideal gas are also simultaneously calculated. Many equations of state have been proposed in the literature with either an empirical, semi- empirical or theoretical basis. Comprehensive reviews can be found in the works of Martin (1979), Gubbins (1983), Anderko (1990), Sandler (1994), Economou and Donohue (1996), Wei and Sadus (2000) and Sengers et al. (2000). The van der Waals equation of state (1873) was the first equation to predict vapour-liquid coexistence. Later, the Redlich-Kwong equation of state (Redlich and Kwong, 1949) improved the accuracy of the van der Waals equation by proposing a temperature dependence for the attractive term. Soave (1972) and Peng and Robinson (1976) proposed additional modifications of the Redlich-Kwong equation to more accurately predict the vapour pressure, liquid density, and equilibria ratios. Guggenheim (1965) and Carnahan and Starling (1969) modified the repulsive term of van der Waals equation of state and obtained more accurate expressions for hard sphere systems. Christoforakos and Franck (1986) modified both the attractive and repulsive terms of van der Waals equation of state. Boublik (1981) extended the Carnahan-Starling hard sphere term to obtain an accurate equation for hard convex geometries. -

“Mining” Water Ice on Mars an Assessment of ISRU Options in Support of Future Human Missions
National Aeronautics and Space Administration “Mining” Water Ice on Mars An Assessment of ISRU Options in Support of Future Human Missions Stephen Hoffman, Alida Andrews, Kevin Watts July 2016 Agenda • Introduction • What kind of water ice are we talking about • Options for accessing the water ice • Drilling Options • “Mining” Options • EMC scenario and requirements • Recommendations and future work Acknowledgement • The authors of this report learned much during the process of researching the technologies and operations associated with drilling into icy deposits and extract water from those deposits. We would like to acknowledge the support and advice provided by the following individuals and their organizations: – Brian Glass, PhD, NASA Ames Research Center – Robert Haehnel, PhD, U.S. Army Corps of Engineers/Cold Regions Research and Engineering Laboratory – Patrick Haggerty, National Science Foundation/Geosciences/Polar Programs – Jennifer Mercer, PhD, National Science Foundation/Geosciences/Polar Programs – Frank Rack, PhD, University of Nebraska-Lincoln – Jason Weale, U.S. Army Corps of Engineers/Cold Regions Research and Engineering Laboratory Mining Water Ice on Mars INTRODUCTION Background • Addendum to M-WIP study, addressing one of the areas not fully covered in this report: accessing and mining water ice if it is present in certain glacier-like forms – The M-WIP report is available at http://mepag.nasa.gov/reports.cfm • The First Landing Site/Exploration Zone Workshop for Human Missions to Mars (October 2015) set the target -

Usgs Water Data
USGS WATER RESOURCES ONLINE PLACES TO START USGS Water home page http://water.usgs.gov/ USGS Water data page http://water.usgs.gov/data/ USGS Publications Warehouse http://pubs.er.usgs.gov/ USGS National Real-Time Water Quality http://nrtwq.usgs.gov/ USGS Water Science Centers http://xx.water.usgs.gov/ (where “xx” is two-letter State code) USGS WATER DATA NWIS-Web http://waterdata.usgs.gov/ NWIS-Web is the general online interface to the USGS National Water Information System (NWIS). Discrete water-sample and time-series data from 1.5 million sites in all 50 States. Results from 5 million water samples with 90 million water-quality results are available from a wide variety of retrieval methods including standard and customized map interfaces. Time-series retrievals include instantaneous measurements back to October 2007, soon to include the longer historical record. BioData http://aquatic.biodata.usgs.gov/ Access to results from more than 15,000 macroinvertebrate, algae, and fish community samples from more than 2,000 sites nationwide. StreamStats http://streamstats.usgs.gov/ssonline.html A map-based tool that allows users to easily obtain streamflow statistics, drainage-basin characteristics, and other information for user-selected sites on streams. Water Quality Portal http://www.waterqualitydata.us/ A cooperative service sponsored by USGS, EPA, and NWQMC that integrates publicly available water-quality data from the USGS NWIS database and the EPA STORET data warehouse. NAWQA Data Warehouse http://water.usgs.gov/nawqa/data.html A collection of chemical, biological, and physical water quality data used in the National Water Quality Assessment (NAWQA) program, drawn partly from NWIS and BioData. -

Liquid-Vapor Equilibrium in a Binary System
Liquid-Vapor Equilibria in Binary Systems1 Purpose The purpose of this experiment is to study a binary liquid-vapor equilibrium of chloroform and acetone. Measurements of liquid and vapor compositions will be made by refractometry. The data will be treated according to equilibrium thermodynamic considerations, which are developed in the theory section. Theory Consider a liquid-gas equilibrium involving more than one species. By definition, an ideal solution is one in which the vapor pressure of a particular component is proportional to the mole fraction of that component in the liquid phase over the entire range of mole fractions. Note that no distinction is made between solute and solvent. The proportionality constant is the vapor pressure of the pure material. Empirically it has been found that in very dilute solutions the vapor pressure of solvent (major component) is proportional to the mole fraction X of the solvent. The proportionality constant is the vapor pressure, po, of the pure solvent. This rule is called Raoult's law: o (1) psolvent = p solvent Xsolvent for Xsolvent = 1 For a truly ideal solution, this law should apply over the entire range of compositions. However, as Xsolvent decreases, a point will generally be reached where the vapor pressure no longer follows the ideal relationship. Similarly, if we consider the solute in an ideal solution, then Eq.(1) should be valid. Experimentally, it is generally found that for dilute real solutions the following relationship is obeyed: psolute=K Xsolute for Xsolute<< 1 (2) where K is a constant but not equal to the vapor pressure of pure solute. -

ESSENTIALS of METEOROLOGY (7Th Ed.) GLOSSARY
ESSENTIALS OF METEOROLOGY (7th ed.) GLOSSARY Chapter 1 Aerosols Tiny suspended solid particles (dust, smoke, etc.) or liquid droplets that enter the atmosphere from either natural or human (anthropogenic) sources, such as the burning of fossil fuels. Sulfur-containing fossil fuels, such as coal, produce sulfate aerosols. Air density The ratio of the mass of a substance to the volume occupied by it. Air density is usually expressed as g/cm3 or kg/m3. Also See Density. Air pressure The pressure exerted by the mass of air above a given point, usually expressed in millibars (mb), inches of (atmospheric mercury (Hg) or in hectopascals (hPa). pressure) Atmosphere The envelope of gases that surround a planet and are held to it by the planet's gravitational attraction. The earth's atmosphere is mainly nitrogen and oxygen. Carbon dioxide (CO2) A colorless, odorless gas whose concentration is about 0.039 percent (390 ppm) in a volume of air near sea level. It is a selective absorber of infrared radiation and, consequently, it is important in the earth's atmospheric greenhouse effect. Solid CO2 is called dry ice. Climate The accumulation of daily and seasonal weather events over a long period of time. Front The transition zone between two distinct air masses. Hurricane A tropical cyclone having winds in excess of 64 knots (74 mi/hr). Ionosphere An electrified region of the upper atmosphere where fairly large concentrations of ions and free electrons exist. Lapse rate The rate at which an atmospheric variable (usually temperature) decreases with height. (See Environmental lapse rate.) Mesosphere The atmospheric layer between the stratosphere and the thermosphere. -

Hydraulics Manual Glossary G - 3
Glossary G - 1 GLOSSARY OF HIGHWAY-RELATED DRAINAGE TERMS (Reprinted from the 1999 edition of the American Association of State Highway and Transportation Officials Model Drainage Manual) G.1 Introduction This Glossary is divided into three parts: · Introduction, · Glossary, and · References. It is not intended that all the terms in this Glossary be rigorously accurate or complete. Realistically, this is impossible. Depending on the circumstance, a particular term may have several meanings; this can never change. The primary purpose of this Glossary is to define the terms found in the Highway Drainage Guidelines and Model Drainage Manual in a manner that makes them easier to interpret and understand. A lesser purpose is to provide a compendium of terms that will be useful for both the novice as well as the more experienced hydraulics engineer. This Glossary may also help those who are unfamiliar with highway drainage design to become more understanding and appreciative of this complex science as well as facilitate communication between the highway hydraulics engineer and others. Where readily available, the source of a definition has been referenced. For clarity or format purposes, cited definitions may have some additional verbiage contained in double brackets [ ]. Conversely, three “dots” (...) are used to indicate where some parts of a cited definition were eliminated. Also, as might be expected, different sources were found to use different hyphenation and terminology practices for the same words. Insignificant changes in this regard were made to some cited references and elsewhere to gain uniformity for the terms contained in this Glossary: as an example, “groundwater” vice “ground-water” or “ground water,” and “cross section area” vice “cross-sectional area.” Cited definitions were taken primarily from two sources: W.B. -

Glossary of Terms
GLOSSARY OF TERMS For the purpose of this Handbook, the following definitions and abbreviations shall apply. Although all of the definitions and abbreviations listed below may have not been used in this Handbook, the additional terminology is provided to assist the user of Handbook in understanding technical terminology associated with Drainage Improvement Projects and the associated regulations. Program-specific terms have been defined separately for each program and are contained in pertinent sub-sections of Section 2 of this handbook. ACRONYMS ASTM American Society for Testing Materials CBBEL Christopher B. Burke Engineering, Ltd. COE United States Army Corps of Engineers EPA Environmental Protection Agency IDEM Indiana Department of Environmental Management IDNR Indiana Department of Natural Resources NRCS USDA-Natural Resources Conservation Service SWCD Soil and Water Conservation District USDA United States Department of Agriculture USFWS United States Fish and Wildlife Service DEFINITIONS AASHTO Classification. The official classification of soil materials and soil aggregate mixtures for highway construction used by the American Association of State Highway and Transportation Officials. Abutment. The sloping sides of a valley that supports the ends of a dam. Acre-Foot. The volume of water that will cover 1 acre to a depth of 1 ft. Aggregate. (1) The sand and gravel portion of concrete (65 to 75% by volume), the rest being cement and water. Fine aggregate contains particles ranging from 1/4 in. down to that retained on a 200-mesh screen. Coarse aggregate ranges from 1/4 in. up to l½ in. (2) That which is installed for the purpose of changing drainage characteristics. -

TABLE A-2 Properties of Saturated Water (Liquid–Vapor): Temperature Table Specific Volume Internal Energy Enthalpy Entropy # M3/Kg Kj/Kg Kj/Kg Kj/Kg K Sat
720 Tables in SI Units TABLE A-2 Properties of Saturated Water (Liquid–Vapor): Temperature Table Specific Volume Internal Energy Enthalpy Entropy # m3/kg kJ/kg kJ/kg kJ/kg K Sat. Sat. Sat. Sat. Sat. Sat. Sat. Sat. Temp. Press. Liquid Vapor Liquid Vapor Liquid Evap. Vapor Liquid Vapor Temp. Њ v ϫ 3 v Њ C bar f 10 g uf ug hf hfg hg sf sg C O 2 .01 0.00611 1.0002 206.136 0.00 2375.3 0.01 2501.3 2501.4 0.0000 9.1562 .01 H 4 0.00813 1.0001 157.232 16.77 2380.9 16.78 2491.9 2508.7 0.0610 9.0514 4 5 0.00872 1.0001 147.120 20.97 2382.3 20.98 2489.6 2510.6 0.0761 9.0257 5 6 0.00935 1.0001 137.734 25.19 2383.6 25.20 2487.2 2512.4 0.0912 9.0003 6 8 0.01072 1.0002 120.917 33.59 2386.4 33.60 2482.5 2516.1 0.1212 8.9501 8 10 0.01228 1.0004 106.379 42.00 2389.2 42.01 2477.7 2519.8 0.1510 8.9008 10 11 0.01312 1.0004 99.857 46.20 2390.5 46.20 2475.4 2521.6 0.1658 8.8765 11 12 0.01402 1.0005 93.784 50.41 2391.9 50.41 2473.0 2523.4 0.1806 8.8524 12 13 0.01497 1.0007 88.124 54.60 2393.3 54.60 2470.7 2525.3 0.1953 8.8285 13 14 0.01598 1.0008 82.848 58.79 2394.7 58.80 2468.3 2527.1 0.2099 8.8048 14 15 0.01705 1.0009 77.926 62.99 2396.1 62.99 2465.9 2528.9 0.2245 8.7814 15 16 0.01818 1.0011 73.333 67.18 2397.4 67.19 2463.6 2530.8 0.2390 8.7582 16 17 0.01938 1.0012 69.044 71.38 2398.8 71.38 2461.2 2532.6 0.2535 8.7351 17 18 0.02064 1.0014 65.038 75.57 2400.2 75.58 2458.8 2534.4 0.2679 8.7123 18 19 0.02198 1.0016 61.293 79.76 2401.6 79.77 2456.5 2536.2 0.2823 8.6897 19 20 0.02339 1.0018 57.791 83.95 2402.9 83.96 2454.1 2538.1 0.2966 8.6672 20 21 0.02487 1.0020 -

Glossary of Terms
Glossary of English/Spanish Superfund & WQARF Terms (Note: You may access the bookmark menu at the left to navigate this document more efficiently.) Any ADEQ translation or communication in a language other than English is unofficial and not binding on the State of Arizona. Cualquier traducción o comunicado de ADEQ en un idioma diferente al inglés no es oficial y no sujetará al Estado de Arizona a ninguna obligación jurídica. A Absorption: The passage of one substance into or through another. Absorción: Absorción es el paso de una sustancia a través de otra. Acre-foot: A quantity or volume of water covering one acre to a depth of one foot; equal to 43,560 cubic feet or 325,851 gallons. Acre-pie: Una cantidad o volumen de agua que cubre un acre a una profundidad de un pie; es un equivalente a 43.560 pies cúbicos o 325.851 galones. Activated Carbon: Adsorptive particles or granules of carbon usually obtained by heating carbon (such as wood). These particles or granules have a high capacity to selectively remove certain trace and soluble materials from water. Carbono Activo: Partículas or gránulos de carbono que se obtienen generalmente por medio del calentamiento de carbono (como la madera). Estas partículas o gránulos tienen una alta capacidad de eliminar selectivamente ciertos rastros y materiales solubles del agua. Acute: Occurring over a short period of time; used to describe brief exposures and effects which appear promptly after exposure. Grave: Ocurre durante corto tiempo; se usa para describir breves exposiciones y los efectos que aparecen rapidamente después de una sola exposición. -

An Analysis of Water Data Systems to Inform the Open Water Data Initiative1
JOURNAL OF THE AMERICAN WATER RESOURCES ASSOCIATION Vol. 52, No. 4 AMERICAN WATER RESOURCES ASSOCIATION August 2016 AN ANALYSIS OF WATER DATA SYSTEMS TO INFORM THE OPEN WATER DATA INITIATIVE1 David Blodgett, Emily Read, Jessica Lucido, Tad Slawecki, and Dwane Young2 ABSTRACT: Improving access to data and fostering open exchange of water information is foundational to solv- ing water resources issues. In this vein, the Department of the Interior’s Assistant Secretary for Water and Science put forward the charge to undertake an Open Water Data Initiative (OWDI) that would prioritize and accelerate work toward better water data infrastructure. The goal of the OWDI is to build out the Open Water Web (OWW). We therefore considered the OWW in terms of four conceptual functions: water data cataloging, water data as a service, enriching water data, and community for water data. To describe the current state of the OWW and identify areas needing improvement, we conducted an analysis of existing systems using a stan- dard model for describing distributed systems and their business requirements. Our analysis considered three OWDI-focused use cases—flooding, drought, and contaminant transport—and then examined the landscape of other existing applications that support the Open Water Web. The analysis, which includes a discussion of observed successful practices of cataloging, serving, enriching, and building community around water resources data, demonstrates that we have made significant progress toward the needed infrastructure, although chal- lenges remain. The further development of the OWW can be greatly informed by the interpretation and findings of our analysis. (KEY TERMS: data management; public participation; hydrologic cycle; geospatial analysis; open data; network linked asset.) Blodgett, David, Emily Read, Jessica Lucido, Tad Slawecki, and Dwane Young, 2016.